iFluor® 625 maleimide
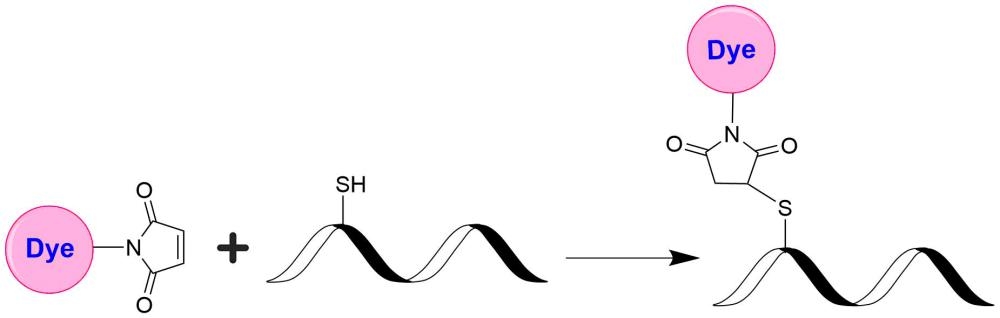
iFluor® 625 maleimide selectively reacts with thiol group of a biomolecule. It is widely used to label reduced antibodies and other thiol-containing biomolecules such as peptides and thiol-modified oligos. iFluor®625 is a fluorophore from the iFluor® family, which is known for its bright red fluorescence and compatibility with various fluorescence techniques and instruments. When excited with light in the red range (around 600 to 650 nm), iFluor® 625 emits strong red fluorescence. It can also be well excited with red lasers at 633 and 647 nm. iFluor® 625 can be conjugated to a variety of biomolecules, including antibodies, proteins, nucleic acids, and small molecules, enabling their visualization and tracking in cells and tissues. It is commonly used in fluorescence microscopy, immunohistochemistry, flow cytometry, and other fluorescence-based assays.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1423 | 1 mg | Price |
Physical properties
| Molecular weight | 1065.85 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 624 |
| Emission (nm) | 640 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 5, 2026

