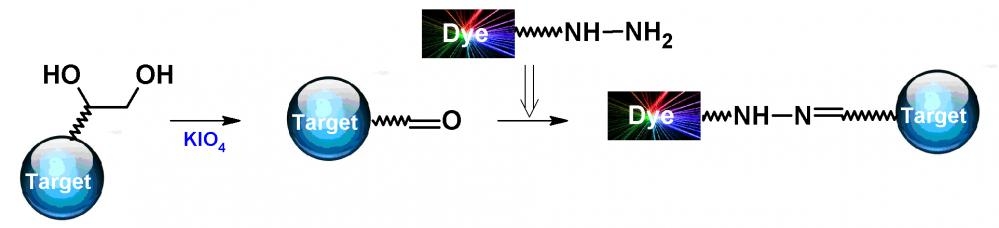
iFluor® 790 hydrazide
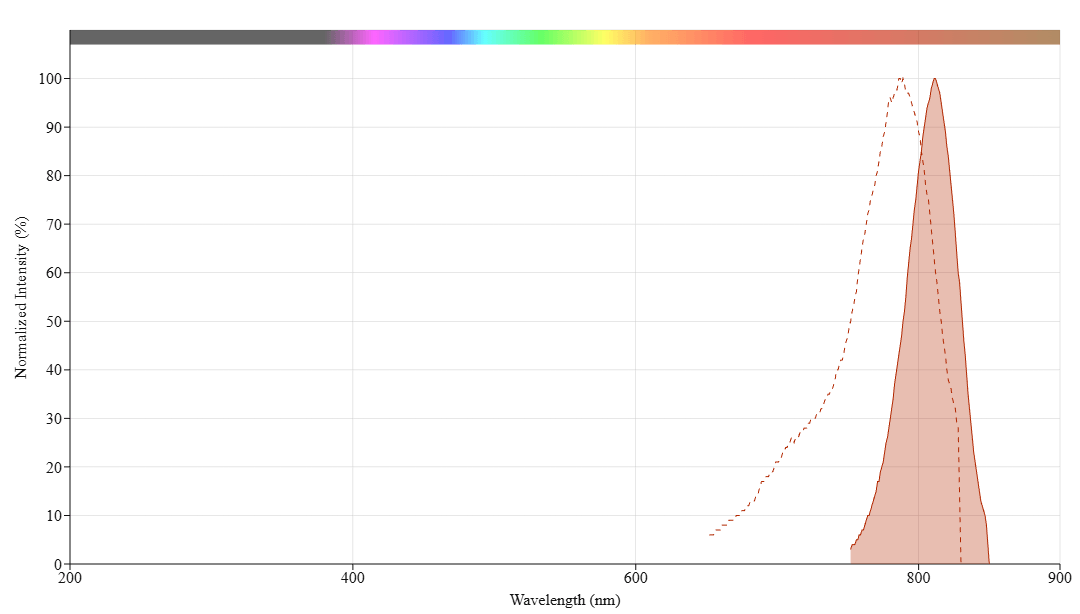
In vivo fluorescence imaging uses a sensitive camera to detect the fluorescence emission from fluorophores in whole-body living small animals. To overcome the photon attenuation in living tissue, fluorophores with long emission at the near-infrared (NIR) region are generally preferred, including widely used small indocarbocyanine dyes. Recent advances in imaging strategies and reporter techniques for in vivo fluorescence imaging include novel approaches to improve the specificity and affinity of the probes and to modulate and amplify the signal at target sites for enhanced sensitivity. Further emerging developments aim to achieve high-resolution, multimodality, and lifetime-based in vivo fluorescence imaging. Our iFluor® 790 is designed to label proteins and other biomolecules with near-infrared fluorescence. Conjugates prepared with iFluor® 790 have excitation and emission spectra similar to that of indocyanine green (ICG) and the IRDye® 800, with 787/812 nm excitation/emission maxima. iFluor® 790 dye emission is well separated from commonly used far-red fluorophores such as Cy5, Cy7, or allophycocyanin (APC), facilitating multicolor analysis. This fluorophore is also useful for small animal in-vivo imaging applications or other imaging applications requiring NIR detections, such as the two-color western applications with the LI-COR® Odyssey® infrared imaging system.
Example protocol
AT A GLANCE
Important notes
It is important to store at <-15 °C and should be stored in cool, dark place.
It can be used within 12 months from the date of receipt.
PREPARATION OF WORKING SOLUTION
iFluor™ 790 hydrazide working solution:
Add water to make iFluor™ 790 hydrazide working solution of desired concentration.
Calculators
Common stock solution preparation
Table 1. Volume of Water needed to reconstitute specific mass of iFluor® 790 hydrazide to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 86.244 µL | 431.22 µL | 862.441 µL | 4.312 mL | 8.624 mL |
| 5 mM | 17.249 µL | 86.244 µL | 172.488 µL | 862.441 µL | 1.725 mL |
| 10 mM | 8.624 µL | 43.122 µL | 86.244 µL | 431.22 µL | 862.441 µL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 hydrazide | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 405 hydrazide | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| iFluor® 488 hydrazide | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| iFluor® 555 hydrazide | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| iFluor® 647 hydrazide | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| iFluor® 680 hydrazide | 684 | 701 | 2200001 | 0.231 | 0.097 | 0.094 |
| iFluor® 700 hydrazide | 690 | 713 | 2200001 | 0.231 | 0.09 | 0.04 |
| iFluor® 750 hydrazide | 757 | 779 | 2750001 | 0.121 | 0.044 | 0.039 |
| iFluor® 790 Styramide *Superior Replacement for Alexa Fluor 790 tyramide* | 787 | 812 | 2500001 | 0.131 | 0.1 | 0.09 |
Citations
View all 1 citations: Citation Explorer
Nanovesicle delivery to the liver via retinol binding protein and platelet-derived growth factor receptors: how targeting ligands affect biodistribution
Authors: Hsu, Ching-Yun and Chen, Chun-Han and Aljuffali, Ibrahim A and Dai, You-Shan and Fang, Jia-You
Journal: Nanomedicine (2017)
Authors: Hsu, Ching-Yun and Chen, Chun-Han and Aljuffali, Ibrahim A and Dai, You-Shan and Fang, Jia-You
Journal: Nanomedicine (2017)
References
View all 18 references: Citation Explorer
A target cell-specific activatable fluorescence probe for in vivo molecular imaging of cancer based on a self-quenched avidin-rhodamine conjugate
Authors: Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Shin IS, Paik CH, Choyke PL, Kobayashi H.
Journal: Cancer Res (2007): 2791
Authors: Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Shin IS, Paik CH, Choyke PL, Kobayashi H.
Journal: Cancer Res (2007): 2791
Fluorescence imaging in vivo: recent advances
Authors: Rao J, Dragulescu-Andrasi A, Yao H.
Journal: Curr Opin Biotechnol (2007): 17
Authors: Rao J, Dragulescu-Andrasi A, Yao H.
Journal: Curr Opin Biotechnol (2007): 17
Ex vivo fluorescence imaging of normal and malignant urothelial cells to enhance early diagnosis
Authors: Steenkeste K, Lecart S, Deniset A, Pernot P, Eschwege P, Ferlicot S, Leveque-Fort S, Bri and et R, Fontaine-Aupart MP.
Journal: Photochem Photobiol (2007): 1157
Authors: Steenkeste K, Lecart S, Deniset A, Pernot P, Eschwege P, Ferlicot S, Leveque-Fort S, Bri and et R, Fontaine-Aupart MP.
Journal: Photochem Photobiol (2007): 1157
In vivo monitoring the fate of Cy5.5-Tat labeled T lymphocytes by quantitative near-infrared fluorescence imaging during acute brain inflammation in a rat model of experimental autoimmune encephalomyelitis
Authors: Berger C, Gremlich HU, Schmidt P, Cannet C, Kneuer R, Hiest and P, Rausch M, Rudin M.
Journal: J Immunol Methods (2007): 65
Authors: Berger C, Gremlich HU, Schmidt P, Cannet C, Kneuer R, Hiest and P, Rausch M, Rudin M.
Journal: J Immunol Methods (2007): 65
A protocol for imaging alternative splicing regulation in vivo using fluorescence reporters in transgenic mice
Authors: Bonano VI, Oltean S, Garcia-Blanco MA.
Journal: Nat Protoc (2007): 2166
Authors: Bonano VI, Oltean S, Garcia-Blanco MA.
Journal: Nat Protoc (2007): 2166
Page updated on March 11, 2025