iFluor® 790 hydrazide
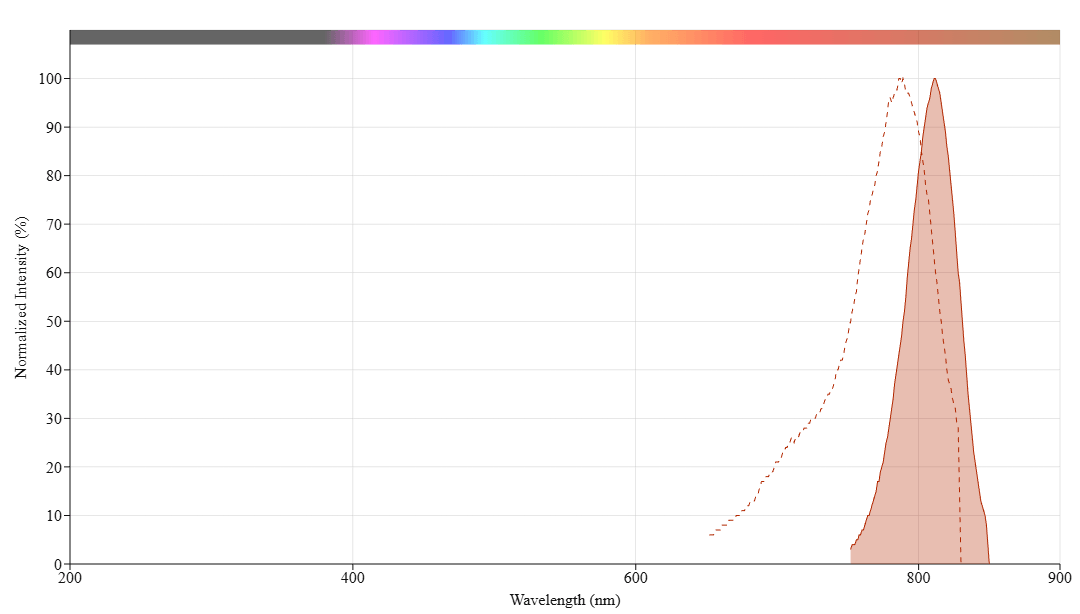
In vivo fluorescence imaging uses a sensitive camera to detect the fluorescence emission from fluorophores in whole-body living small animals. To overcome the photon attenuation in living tissue, fluorophores with long emission at the near-infrared (NIR) region are generally preferred, including widely used small indocarbocyanine dyes. Recent advances in imaging strategies and reporter techniques for in vivo fluorescence imaging include novel approaches to improve the specificity and affinity of the probes and to modulate and amplify the signal at target sites for enhanced sensitivity. Further emerging developments aim to achieve high-resolution, multimodality, and lifetime-based in vivo fluorescence imaging. Our iFluor® 790 is designed to label proteins and other biomolecules with near-infrared fluorescence. Conjugates prepared with iFluor® 790 have excitation and emission spectra similar to that of indocyanine green (ICG) and the IRDye® 800, with 787/812 nm excitation/emission maxima. iFluor® 790 dye emission is well separated from commonly used far-red fluorophores such as Cy5, Cy7, or allophycocyanin (APC), facilitating multicolor analysis. This fluorophore is also useful for small animal in-vivo imaging applications or other imaging applications requiring NIR detections, such as the two-color western applications with the LI-COR® Odyssey® infrared imaging system.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1364 | 1 mg | Price |
Physical properties
| Molecular weight | 1159.50 |
| Solvent | Water |
Spectral properties
| Correction factor (260 nm) | 0.1 |
| Correction factor (280 nm) | 0.09 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 787 |
| Emission (nm) | 812 |
| Quantum yield | 0.13 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 783 nm |
| Emission | 814 nm |
| Cutoff | 790 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 12, 2026