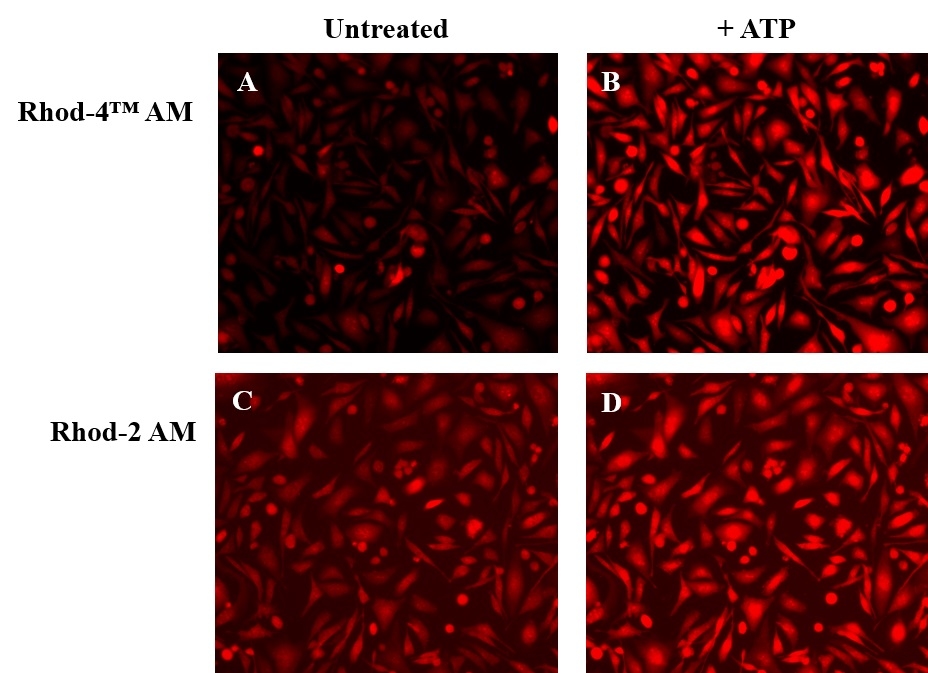
Rhod-4™, AM
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 2 to 5 mM stock solution of Rhod-4™ AM in high-quality, anhydrous DMSO.
PREPARATION OF WORKING SOLUTION
On the day of the experiment, either dissolve Rhod-4™ AM in DMSO or thaw an aliquot of the indicator stock solution to room temperature.
Prepare a 2 to 20 µM Rhod-4™ AM working solution in a buffer of your choice (e.g., Hanks and Hepes buffer) with 0.04% Pluronic® F-127. For most cell lines, Rhod-4™ AM at a final concentration of 4-5 μM is recommended. The exact concentration of indicators required for cell loading must be determined empirically.
Note: The nonionic detergent Pluronic® F-127 is sometimes used to increase the aqueous solubility of Rhod-4™ AM. A variety of Pluronic® F-127 solutions can be purchased from AAT Bioquest.
Note: If your cells contain organic anion-transporters, probenecid (1-2 mM) may be added to the dye working solution (final in well concentration will be 0.5-1 mM) to reduce leakage of the de-esterified indicators. A variety of ReadiUse™ Probenecid products, including water-soluble, sodium salt, and stabilized solutions, can be purchased from AAT Bioquest.
SAMPLE EXPERIMENTAL PROTOCOL
Following is our recommended protocol for loading AM esters into live cells. This protocol only provides a guideline and should be modified according to your specific needs.
- Prepare cells in growth medium overnight.
On the next day, add 1X Rhod-4™ AM working solution to your cell plate.
Note: If your compound(s) interfere with the serum, replace the growth medium with fresh HHBS buffer before dye-loading.
Incubate the dye-loaded plate in a cell incubator at 37 °C for 30 to 60 minutes.
Note: Incubating the dye for longer than 1 hour can improve signal intensities in certain cell lines.
- Replace the dye working solution with HHBS or buffer of your choice (containing an anion transporter inhibitor, such as 1 mM probenecid, if applicable) to remove any excess probes.
- Add the stimulant as desired and simultaneously measure fluorescence using either a fluorescence microscope equipped with a TRITC filter set or a fluorescence plate reader containing a programmable liquid handling system such as an FDSS, FLIPR, or FlexStation, at Ex/Em = 540/590 nm cutoff 570 nm.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 98.429 µL | 492.145 µL | 984.291 µL | 4.921 mL | 9.843 mL |
| 5 mM | 19.686 µL | 98.429 µL | 196.858 µL | 984.291 µL | 1.969 mL |
| 10 mM | 9.843 µL | 49.215 µL | 98.429 µL | 492.145 µL | 984.291 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Quantum yield |
| Rhod-4™ alkyne | 523 | 551 | 0.11 |
| Rhod-4™ amine | 523 | 551 | 0.11 |
| Rhod-4™ azide | 523 | 551 | 0.11 |
| Rhod-4™ maleimide | 523 | 551 | 0.11 |
| Fluo-4 AM *Ultrapure Grade* *CAS 273221-67-3* | 495 | 528 | 0.161 |
| Rhod-2, AM *CAS#: 145037-81-6* | 553 | 577 | 0.11 |
| Rhod-2, AM *UltraPure Grade* *CAS#: 145037-81-6* | 553 | 577 | 0.11 |
| Rhod-5N, AM | 557 | 580 | - |
| Rhod-FF, AM | 553 | 577 | - |
Citations
Authors: Rahman, Fasih A and Yap, Jasmine M Friedrich and Joseph, Tyler M and Adam, Amanda M and Chapman, Sarah M and Quadrilatero, Joe
Journal: Cell Communication and Signaling: CCS (2025): 158
Authors: Pozo, Maria R and Heinson, Yuli W and Chua, Christianne J and Entcheva, Emilia
Journal: Biophysical Journal (2025)
Authors: Steiner, Katharina and Humpel, Christian
Journal: Frontiers in Bioscience-Landmark (2024): 232
Authors: Liu, Wei and Han, Julie L and Tomek, Jakub and Bub, Gil and Entcheva, Emilia
Journal: ACS Photonics (2023)
Authors: Smith, Alyson S and Ankam, Soneela and Farhy, Chen and Fiengo, Lorenzo and Basa, Ranor CB and Gordon, Kara L and Martin, Charles T and Terskikh, Alexey V and Jordan-Sciutto, Kelly L and Price, Jeffrey H and others,
Journal: Journal of Pharmacological and Toxicological Methods (2022): 107157
References
Authors: Territo PR, Heil J, Bose S, Evans FJ, Balaban RS.
Journal: Appl Spectrosc (2007): 138
Authors: Stamm C, del Nido PJ.
Journal: Thorac Cardiovasc Surg (2004): 127
Authors: Martin VV, Beierlein M, Morgan JL, Rothe A, Gee KR.
Journal: Cell Calcium (2004): 509
Authors: Bednar B, Cunningham ME, Kiss L, Cheng G, McCauley JA, Liverton NJ, Koblan KS.
Journal: J Neurosci Methods (2004): 247
Authors: Stamm C, Friehs I, Choi YH, Zurakowski D, McGowan FX, del Nido PJ.
Journal: Cardiovasc Res (2003): 695




![GPR157 couples with Gq-class of the heterotrimeric G-proteins. (A–C) Plasmids expressing indicated protein were transfected into U-2 OS cells. Rhod-4, a fluorescent calcium indicator, were used to assess changes in [Ca<sup>2+</sup>]i. Application of Ionomycin, a calcium ionopohore, to cells after experiments confirmed almost uniform uptake of Rhod-4 in these cells. (D–F) Fluorescent intensity of Rhod4 in GFP-positive cells. Mean ± s.e.m. Data were obtained from 3 independent experiments (more than 40 cells). **p < 0.01, ***p < 0.001. Scale bar: 10 μm. Source: <strong>The G protein-coupled receptor GPR157 regulates neuronal differentiation of radial glial progenitors through the Gq-IP3 pathway </strong>by Takeo et al., <em>Scientific Reports</em>, May 2016.](/_next/image?url=https%3A%2F%2Fimages.aatbio.com%2Fproducts%2Ffigures-and-data%2Frhod-4-am%2Ffigure-for-rhod-4-am_NeyL7.jpg&w=128&q=25)

