ReadiLink™ xtra Rapid Cy7 Antibody Labeling Kit *BSA-Compatible*
ReadiLink™ xtra rapid antibody labeling kits require essentially only 2 simple mixing steps without a column purification needed. Specially formulated Cy7 used in this ReadiLink™ kit is quite stable and shows good reactivity and selectivity with antibodies. The kit has all the essential components for labeling ~2x50 ug antibody. Each of the two vials of specially formulated Cy7 dye provided in the kit is optimized for labeling ~50 µg antibody. ReadiLink™ xtra Cy7 rapid antibody labeling kit provides a convenient and robust method to label monoclonal and polyclonal antibodies with NIR fluorescent Cy7 fluorophore. Cy7 is one of the most used fluorophores for labeling antibodies. 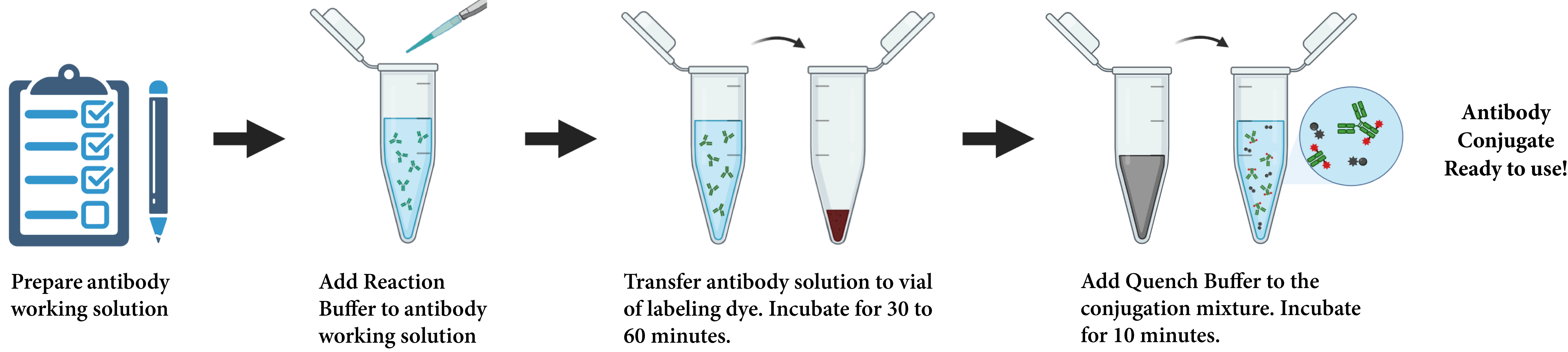

Figure 1. Overview of the ReadiLink™ xtra Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.
Example protocol
AT A GLANCE
Important
Warm all the components and centrifuge the vials briefly before opening, and immediately prepare the required solutions before starting your conjugation. The following protocol is for recommendation.PREPARATION OF WORKING SOLUTION
Protein working solution (Solution A)
For labeling 50 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 5 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 50 µL of the target protein solution.Note If you have a different protein concentration, adjust the protein volume accordingly to make ~50 µg of protein available for your labeling reaction.
Note For labeling 100 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 10 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 100 µL of the target protein solution.
Note The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2 - 7.4; if the protein is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra-0.5, Ultracel-10 Membrane, 10 kDa (cat# UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) with 0.1 to 0.5 % will be labeled well.
Note For optimal labeling efficiency, a final protein concentration range of 1 - 2 mg/mL is recommended, with a significantly reduced conjugation efficiency at less than 1 mg/mL.
SAMPLE EXPERIMENTAL PROTOCOL
Run conjugation reaction
- Add the protein working solution (Solution A) to ONE vial of labeling dye (Component A), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Note If labeling 100 µg of protein, use both vials (Component A) of labeling dye by dividing the 100 µg of protein into 2 x 50 µg of protein and reacting each 50 µg of protein with one vial of labeling dye. Then combine both vials for the next step. - Keep the conjugation reaction mixture at room temperature for 30 - 60 minutes.
Note The conjugation reaction mixture can be rotated or shaken for longer time if desired.
Stop Conjugation reaction
- Add 5 µL (for 50 µg protein) or 10 µL (for 100 µg protein) which is 10% of the total reaction volume of TQ™-Dyed Quench Buffer (Component C) into the conjugation reaction mixture; mix well.
- Incubate at room temperature for 10 minutes. The labeled protein (antibody) is now ready to use.
Storage of Protein Conjugate
The protein conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). For longer storage, the protein conjugates could be lyophilized or divided into single-used aliquots and stored at ≤ –20 °C.Spectrum
Product family
References
View all 48 references: Citation Explorer
Near-infrared/pH dual-responsive nanocomplexes for targeted imaging and chemo/gene/photothermal tri-therapies of non-small cell lung cancer.
Authors: Li, Ziying and Zhu, Lisheng and Liu, Weiqun and Zheng, Yilin and Li, Xudong and Ye, Jinxiang and Li, Bifei and Chen, Haijun and Gao, Yu
Journal: Acta biomaterialia (2020): 242-259
Authors: Li, Ziying and Zhu, Lisheng and Liu, Weiqun and Zheng, Yilin and Li, Xudong and Ye, Jinxiang and Li, Bifei and Chen, Haijun and Gao, Yu
Journal: Acta biomaterialia (2020): 242-259
PI3KC3 complex subunit NRBF2 is required for apoptotic cell clearance to restrict intestinal inflammation.
Authors: Wu, Ming-Yue and Liu, Le and Wang, Er-Jin and Xiao, Hai-Tao and Cai, Cui-Zan and Wang, Jing and Su, Huanxing and Wang, Yitao and Tan, Jieqiong and Zhang, Zhuohua and Wang, Juan and Yao, Maojing and Ouyang, De-Fang and Yue, Zhenyu and Li, Min and Chen, Ye and Bian, Zhao-Xiang and Lu, Jia-Hong
Journal: Autophagy (2020): 1-16
Authors: Wu, Ming-Yue and Liu, Le and Wang, Er-Jin and Xiao, Hai-Tao and Cai, Cui-Zan and Wang, Jing and Su, Huanxing and Wang, Yitao and Tan, Jieqiong and Zhang, Zhuohua and Wang, Juan and Yao, Maojing and Ouyang, De-Fang and Yue, Zhenyu and Li, Min and Chen, Ye and Bian, Zhao-Xiang and Lu, Jia-Hong
Journal: Autophagy (2020): 1-16
Quantitative, real-time in vivo tracking of magnetic nanoparticles using multispectral optoacoustic tomography (MSOT) imaging.
Authors: Anani, Tareq and Brannen, Andrew and Panizzi, Peter and Duin, Evert C and David, Allan E
Journal: Journal of pharmaceutical and biomedical analysis (2020): 112951
Authors: Anani, Tareq and Brannen, Andrew and Panizzi, Peter and Duin, Evert C and David, Allan E
Journal: Journal of pharmaceutical and biomedical analysis (2020): 112951
Real-time monitoring and accurate diagnosis of drug-induced hepatotoxicity in vivo by ratio-fluorescence and photoacoustic imaging of peroxynitrite.
Authors: Zhuang, Hongjun and Li, Benhao and Zhao, Mengyao and Wei, Peng and Yuan, Wei and Zhang, Mengfan and Han, Xuemin and Chen, Yin and Yi, Tao
Journal: Nanoscale (2020)
Authors: Zhuang, Hongjun and Li, Benhao and Zhao, Mengyao and Wei, Peng and Yuan, Wei and Zhang, Mengfan and Han, Xuemin and Chen, Yin and Yi, Tao
Journal: Nanoscale (2020)
Supramolecular Nanodiscs Self-Assembled from Non-Ionic Heptamethine Cyanine for Imaging-Guided Cancer Photothermal Therapy.
Authors: Mu, Xueluer and Lu, Yingxi and Wu, Fapu and Wei, Yuhan and Ma, Huihui and Zhao, Yingjie and Sun, Jing and Liu, Shaofeng and Zhou, Xianfeng and Li, Zhibo
Journal: Advanced materials (Deerfield Beach, Fla.) (2020): e1906711
Authors: Mu, Xueluer and Lu, Yingxi and Wu, Fapu and Wei, Yuhan and Ma, Huihui and Zhao, Yingjie and Sun, Jing and Liu, Shaofeng and Zhou, Xianfeng and Li, Zhibo
Journal: Advanced materials (Deerfield Beach, Fla.) (2020): e1906711
Page updated on April 15, 2025




