ReadiLink™ xtra Rapid Cy5 Antibody Labeling Kit
BSA-Compatible
ReadiLink™ xtra rapid antibody labeling kits require essentially only 2 simple mixing steps without a column purification needed. Specially formulated Cy5 used in this ReadiLink™ kit is quite stable and shows good reactivity and selectivity with antibodies. The kit has all the essential components for labeling ~2x50 ug antibody. Each of the two vials of specially formulated Cy5 dye provided in the kit is optimized for labeling ~50 µg antibody. ReadiLink™ xtra Cy5 rapid antibody labeling kit provides a convenient and robust method to label monoclonal and polyclonal antibodies with the red fluorescent Cy5 fluorophore. Cy5 is one of the most used fluorophores for labeling antibodies.
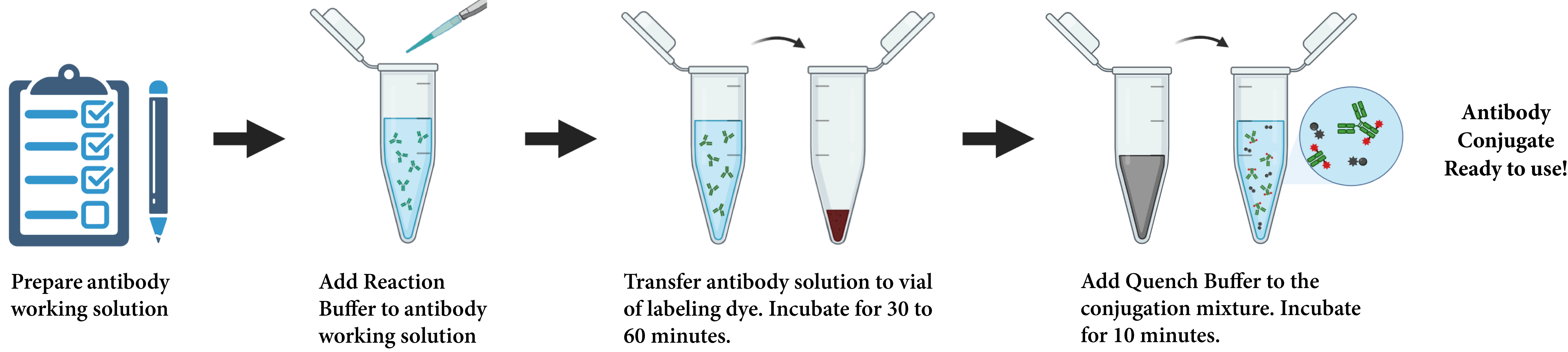
Figure 1. Overview of the ReadiLink™ xtra Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1972 | 2 Labelings | Price |
Spectral properties
| Correction factor (260 nm) | 0.02 |
| Correction factor (280 nm) | 0.03 |
| Correction factor (482 nm) | 0.009 |
| Correction factor (565 nm) | 0.09 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 651 |
| Emission (nm) | 670 |
| Quantum yield | 0.27 1 , 0.42 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 31, 2026

