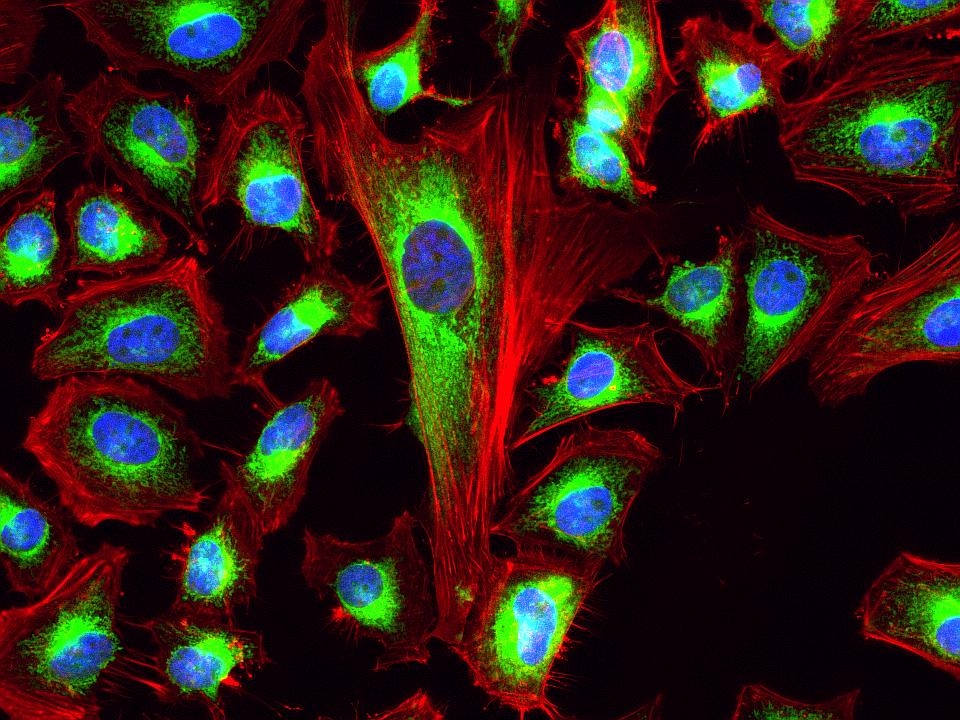
Phalloidin Conjugates
iFluor® 633
This deep red fluorescent phalloidin conjugate (equivalent to Alexa Fluor® 633-labeled phalloidin) selectively binds to F-actins. Used at nanomolar concentrations, phalloidin derivatives are convenient probes for labeling, identifying and quantitating F-actins in formaldehyde-fixed and permeabilized tissue sections, cell cultures or cell-free experiments. Phalloidin binds to actin filaments much more tightly than to actin monomers, leading to a decrease in the rate constant for the dissociation of actin subunits from filament ends, essentially stabilizing actin filaments through the prevention of filament depolymerization. Moreover, phalloidin is found to inhibit the ATP hydrolysis activity of F-actin. Phalloidin functions differently at various concentrations in cells. When introduced into the cytoplasm at low concentrations, phalloidin recruits the less polymerized forms of cytoplasmic actin as well as filamin into stable "islands" of aggregated actin polymers, yet it does not interfere with stress fibers, i.e. thick bundles of microfilaments. The property of phalloidin is a useful tool for investigating the distribution of F-actin in cells by labeling phalloidin with fluorescent analogs and using them to stain actin filaments for light microscopy. Fluorescent derivatives of phalloidin have turned out to be enormously useful in localizing actin filaments in living or fixed cells as well as for visualizing individual actin filaments in vitro. Fluorescent phalloidin derivatives have been used as an important tool in the study of actin networks at high resolution. AAT Bioquest offers a variety of fluorescent phalloidin derivatives with different colors for multicolor imaging applications.
Example protocol
AT A GLANCE
Protocol Summary
- Prepare samples in microplate wells
- Remove liquid from samples in the plate
- Add Phalloidin-iFluor™ 633 Conjugate solution (100 μL/well)
- Stain the cells at room temperature for 20 to 90 minutes
- Wash the cells
- Examine the specimen under microscope with Cy5 filter
Storage and Handling Conditions
The solution should be stable for at least 6 months if store at -20 °C. Protect the fluorescent conjugates from light, and avoid freeze/thaw cycles.Note Phalloidin is toxic, although the amount of toxin present in a vial could be lethal only to a mosquito (LD50 of phalloidin = 2 mg/kg), it should be handled with care.
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
Phalloidin-iFluor™ 633 Conjugate stock solution
Add 30 µL of DMSO into the powder and mix well.PREPARATION OF WORKING SOLUTION
Phalloidin-iFluor™ 633 Conjugate working solution
Add 1 µL of Phalloidin-iFluor™ 633 Conjugate solution to 1 mL of PBS with 1% BSA.Note The stock solution of phalloidin conjugate should be aliquoted and stored at -20 °C. protected from light.
Note Different cell types might be stained differently. The concentration of phalloidin conjugate working solution should be prepared accordingly.
SAMPLE EXPERIMENTAL PROTOCOL
Stain the cells
- Perform formaldehyde fixation. Incubate cells with 3.0–4.0 % formaldehyde in PBS at room temperature for 10–30 minutes.
Note Avoid any methanol containing fixatives since methanol can disrupt actin during the fixation process. The preferred fixative is methanol-free formaldehyde. - Rinse the fixed cells 2–3 times in PBS.
- Optional: Add 0.1% Triton X-100 in PBS into fixed cells for 3 to 5 minutes to increase permeability. Rinse the cells 2–3 times in PBS.
- Add 100 μL/well (96-well plate) of Phalloidin-iFluor™ 633 Conjugate working solution into the fixed cells, and stain the cells at room temperature for 20 to 90 minutes.
- Rinse cells gently with PBS 2 to 3 times to remove excess phalloidin conjugate before plating, sealing and imaging under microscope with Cy5 filter set.
Calculators
Common stock solution preparation
Table 1. Volume of DMSO needed to reconstitute specific mass of Phalloidin-iFluor® 633 Conjugate to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 58.686 µL | 293.429 µL | 586.858 µL | 2.934 mL | 5.869 mL |
| 5 mM | 11.737 µL | 58.686 µL | 117.372 µL | 586.858 µL | 1.174 mL |
| 10 mM | 5.869 µL | 29.343 µL | 58.686 µL | 293.429 µL | 586.858 µL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Alternative formats
| Name | Conjugate |
| Phalloidin-iFluor® 488 Conjugate | iFluor 488 |
| Phalloidin-iFluor® 647 Conjugate | iFluor 647 |
| Phalloidin-iFluor® 594 Conjugate | iFluor 594 |
| Phalloidin-iFluor® 555 Conjugate | iFluor 555 |
| Phalloidin-iFluor® 633 Conjugate | iFluor 633 |
| Phalloidin-iFluor® 532 Conjugate | iFluor 532 |
| Phalloidin-iFluor® 405 Conjugate | iFluor 405 |
| Phalloidin-iFluor® 514 Conjugate | iFluor 514 |
| Phalloidin-iFluor® 350 Conjugate | iFluor 350 |
Show More (15) | |
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| Phalloidin-iFluor® 350 Conjugate | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| Phalloidin-iFluor® 405 Conjugate | 403 | 427 | 370001 | 0.911 | 0.48 | 0.77 |
| Phalloidin-iFluor® 488 Conjugate | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| Phalloidin-iFluor® 514 Conjugate | 511 | 527 | 750001 | 0.831 | 0.265 | 0.116 |
| Phalloidin-iFluor® 532 Conjugate | 537 | 560 | 900001 | 0.681 | 0.26 | 0.16 |
| Phalloidin-iFluor® 555 Conjugate | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| Phalloidin-iFluor® 594 Conjugate | 587 | 603 | 2000001 | 0.531 | 0.05 | 0.04 |
| Phalloidin-iFluor® 647 Conjugate | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| Phalloidin-iFluor® 680 Conjugate | 684 | 701 | 2200001 | 0.231 | 0.097 | 0.094 |
Show More (3) | ||||||
Citations
View all 38 citations: Citation Explorer
Hyphal Als proteins act as CR3 ligands to promote immune responses against Candida albicans
Authors: Zhou, Tingting and Solis, Norma V and Marshall, Michaela and Yao, Qing and Garleb, Rachel and Yang, Mengli and Pearlman, Eric and Filler, Scott G and Liu, Haoping
Journal: Nature Communications (2024): 3926
Authors: Zhou, Tingting and Solis, Norma V and Marshall, Michaela and Yao, Qing and Garleb, Rachel and Yang, Mengli and Pearlman, Eric and Filler, Scott G and Liu, Haoping
Journal: Nature Communications (2024): 3926
Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed $\alpha$-tubulin lactylation
Authors: Li, Lei and Sun, Shuangshuang and Xu, Zhe and He, Liying and Shen, Yihui and Yan, Yuqing and Lv, Xubing and Zheng, Yongjun and Sun, Yadong
Journal: (2024)
Authors: Li, Lei and Sun, Shuangshuang and Xu, Zhe and He, Liying and Shen, Yihui and Yan, Yuqing and Lv, Xubing and Zheng, Yongjun and Sun, Yadong
Journal: (2024)
Regulation of ACE2 isoforms by type 2 inflammation and viral infection in human airway epithelium
Authors: Stocker, N and Radzikowska, U and Wawrzyniak, P and Tan, G and Huang, M and Ding, M and Akdis, CA and Sokolowska, M
Journal: Mucosal Immunology (2023)
Authors: Stocker, N and Radzikowska, U and Wawrzyniak, P and Tan, G and Huang, M and Ding, M and Akdis, CA and Sokolowska, M
Journal: Mucosal Immunology (2023)
Synthetic Collagen-like Polymer That Undergoes a Sol--Gel Transition Triggered by O--N Acyl Migration at Physiological pH
Authors: Ichise, Shinichiro F and Koide, Takaki
Journal: International Journal of Molecular Sciences (2022): 1584
Authors: Ichise, Shinichiro F and Koide, Takaki
Journal: International Journal of Molecular Sciences (2022): 1584
Evaluation of rapid transepithelial electrical resistance (TEER) measurement as a metric of kidney toxicity in a high-throughput microfluidic culture system
Authors: Shaughnessey, Erin M and Kann, Samuel H and Azizgolshani, Hesham and Black, Lauren D and Charest, Joseph L and Vedula, Else M
Journal: Scientific Reports (2022): 1--14
Authors: Shaughnessey, Erin M and Kann, Samuel H and Azizgolshani, Hesham and Black, Lauren D and Charest, Joseph L and Vedula, Else M
Journal: Scientific Reports (2022): 1--14
References
View all 127 references: Citation Explorer
Improved penile histology by phalloidin stain: circular and longitudinal cavernous smooth muscles, dual-endothelium arteries, and erectile dysfunction-associated changes
Authors: Lin G, Qiu X, F and el TM, Albersen M, Wang Z, Lue TF, Lin CS.
Journal: Urology (2011): 970 e1
Authors: Lin G, Qiu X, F and el TM, Albersen M, Wang Z, Lue TF, Lin CS.
Journal: Urology (2011): 970 e1
Phalloidin perturbs the interaction of human non-muscle myosin isoforms 2A and 2C1 with F-actin
Authors: Diensthuber RP, Muller M, Heissler SM, Taft MH, Chizhov I, Manstein DJ.
Journal: FEBS Lett (2011): 767
Authors: Diensthuber RP, Muller M, Heissler SM, Taft MH, Chizhov I, Manstein DJ.
Journal: FEBS Lett (2011): 767
Labeling cytoskeletal F-actin with rhodamine phalloidin or fluorescein phalloidin for imaging
Authors: Chazotte B., undefined
Journal: Cold Spring Harb Protoc (2010): pdb prot4947
Authors: Chazotte B., undefined
Journal: Cold Spring Harb Protoc (2010): pdb prot4947
pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation
Authors: An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM.
Journal: Proc Natl Acad Sci U S A (2010): 20246
Authors: An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM.
Journal: Proc Natl Acad Sci U S A (2010): 20246
Protective effect of bile acid derivatives in phalloidin-induced rat liver toxicity
Authors: Herraez E, Macias RI, Vazquez-Tato J, Hierro C, Monte MJ, Marin JJ.
Journal: Toxicol Appl Pharmacol (2009): 21
Authors: Herraez E, Macias RI, Vazquez-Tato J, Hierro C, Monte MJ, Marin JJ.
Journal: Toxicol Appl Pharmacol (2009): 21
Page updated on October 9, 2024