TUNEL Assay
Red Fluorescence
Cell Meter™ TUNEL apoptosis assay kit provides a robust tool for conveniently detecting apoptosis caused by DNA fragmentation. The assay is non-radioactive and rapid. The TUNEL assay uses the terminal deoxynucleotidyl transferase (TdT) to catalyze the incorporation of TMR-dUTP at the free 3’-hydroxyl ends of the fragmented DNAs. The resulted TMR-labeled DNAs are analyzed by fluorescence microscopy (Cy3 or TRITC filter set). Its red emission can be conveniently multiplexed with GFP labelled targets. Direct incorporation of fluorescent TMR-labeled nucleotides significantly reduces the number of test steps. The kit is optimized to detect apoptosis in fixed cells and formalin-fixed, paraffin-embedded tissue sections.
Example protocol
AT A GLANCE
Protocol summary
- Treat samples as desired
- Fix cells with 4% formaldehyde solution for 30 minutes on ice
- Permeabilize cells with 70% ice-cold ethanol for 60 minutes on ice
- Add TdT staining solution to samples and incubate for 60 minutes at 37 °C
- Monitor the fluorescence intensity using fluorescence microscopy with Cy3 filter set
PREPARATION OF WORKING SOLUTION
TdT staining solution
For one test, Mix the following to make total volume of 51 µL;45 µL TdT Reaction Buffer (Component D)
5 µL CoCl2 (Component C)
0.5 µL TF5-dUTP (Component B)
0.5 µL TdT enzyme (Component A).
Note TdT staining solution should be used promptly.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol can be used as a guideline and should be optimized according to the needs.
Deparaffinization and rehydration protocol
- Treat your samples as desired.
- Wash the cells with buffer of your choice such as PBS containing Ca+2 and Mg+2.
- Fix the cells by adding 100 µL of 4% paraformaldehyde in PBS and incubate the samples for 30 minutes on ice.
- Remove fixation solution and wash cells with PBS.
- Add 100 µL of 70% of ice cold ethanol to cells and incubate the samples for 60 minutes on ice.
Note Cells can be stored at -20 °C at this step for several days before use. - Remove alcohol and wash cells with PBS.
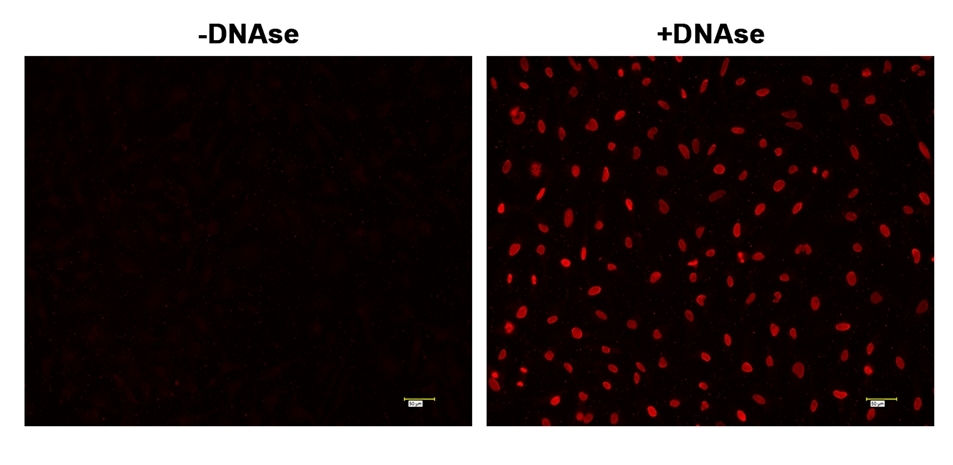
Note For a positive control, incubate fixed cells with 2-5 µg/mL of DNAse in PBS containing Ca+2 and Mg+2 for 60 minutes at 37 °C. Remove the DNAse and wash cells thoroughly and continue with the rest of the protocol. - Add 50 µL of TdT staining solution to the cells and incubate for 60 to 120 minutes at 37 °C.
- Remove TdT working solution and wash cells with PBS.
- Resuspend the cells in PBS and monitor the fluorescence intensity with flow cytometer using 575/26 nm filter (PE channel) or fluorescence microscope with Cy3 filter set.
Protocol for tissue staining
The following protocol can be used as a guideline and should be optimized according to the needs.Deparaffinization and rehydration protocol
- Deparaffinize tissue sections (attached to the microscopic slides) by immersing slides in fresh xylene in a Coplin jar for 5 minutes at room temperature. Repeat one more time. (Total 2 washes)
- Wash the samples by immersing the slides in 100% ethanol for 5 minutes at room temperature in a Coplin jar.
- Rehydrate the samples by immersing the slides through various concentrations of alcohol subsequently (100, 95, 85, 70, 50%) for 5 minutes each at room temperature.
- Wash the samples by immersing the slides in 0.85% NaCl for 5 minutes at room temperature.
- Wash the samples by immersing the slides in PBS for 5 minutes at room temperature. Repeat one more wash. (Total 2 washes)
- Fix the tissue sections by immersing slides in 4% paraformaldehyde solution in PBS for 15-20 minutes at room temperature.
- Wash the samples by immersing the slides in PBS for 5 minutes at room temperature. Repeat one more wash. (Total 2 washes)
- Remove the liquid and place the slides on a flat surface. Treat tissue sections with 100 µL of 20 µg/mL Proteinase K solution. Add enough to cover the entire tissue surface. Incubate slides for 10 minutes at room temperature.
- Wash the samples by immersing the slides in PBS for 5 minutes at room temperature.
- Fix the tissue sections by immersing slides in 4% paraformaldehyde solution in PBS for 15-20 minutes at room temperature.
- Wash the samples by immersing the slides in PBS for 5 minutes at room temperature. Repeat one more wash. (Total 2 washes)
- Optional: For a positive control, incubate fixed samples with 2-5 µg/mL of DNAse in PBS containing Ca+2 and Mg+2 for 60 minutes at 37 °C. Remove the DNAse and wash cells thoroughly with PBS and continue with the rest of the protocol.
- Add 50 µL of TdT staining solution to the samples and incubate for 60 to 120 minutes at 37 °C.
- Remove TdT working solution and wash samples with PBS.
- Add mounting medium with DAPI (AAT Bioquest Cat# 20005) and monitor the fluorescence intensity fluorescence microscope with Cy3 filter set.
Spectrum
Alternative formats
| Name | Sample Type | Fluorescence |
| Cell Meter™ Live Cell TUNEL Apoptosis Assay Kit *Green Fluorescence* | Live Cell | Green |
| Cell Meter™ Live Cell TUNEL Apoptosis Assay Kit *Red Fluorescence* | Live Cell | Red |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Green Fluorescence* | Fixed Cell and Tissue | Green |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Red Fluorescence* | Fixed Cell and Tissue | Red |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Deep Red Fluorescence* | Fixed Cell and Tissue | Deep Red |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Blue Fluorescence* | Fixed Cell and Tissue | Blue |
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Blue Fluorescence* | 411 | 472 | - | - | 0.14 | 0.12 |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Green Fluorescence* | 498 | 517 | 800001 | 0.79001, 0.952 | 0.32 | 0.35 |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Deep Red Fluorescence* | 649 | 663 | 250000 | 0.271 | - | 0.027 |
Citations
View all 11 citations: Citation Explorer
Immuno-protective vesicle-crosslinked hydrogel for allogenic transplantation
Authors: Wang, Yuqian and Huang, Renqi and Lu, Yougong and Liu, Mingqi and Mo, Ran
Journal: Nature Communications (2024): 1--13
Authors: Wang, Yuqian and Huang, Renqi and Lu, Yougong and Liu, Mingqi and Mo, Ran
Journal: Nature Communications (2024): 1--13
In Vitro Effects of Boric Acid on Cell Cycle, Apoptosis, and miRNAs in Medullary Thyroid Cancer Cells
Authors: Y{\i}ld{\i}r{\i}m, Onurcan and Se{\c{c}}me, M{\"u}cahit and Dodurga, Yavuz and Mete, G{\"u}l{\c{c}}in Abban and Fenkci, Semin Melahat
Journal: Biological Trace Element Research (2024): 1--11
Authors: Y{\i}ld{\i}r{\i}m, Onurcan and Se{\c{c}}me, M{\"u}cahit and Dodurga, Yavuz and Mete, G{\"u}l{\c{c}}in Abban and Fenkci, Semin Melahat
Journal: Biological Trace Element Research (2024): 1--11
Antifungal Activity of Cedrol from Cunninghamia lanceolate var. konishii against Phellinus noxius and Its Mechanism
Authors: Hsiao, Wen-Wei and Lau, Ka-Man and Chien, Shih-Chang and Chu, Fang-Hua and Chung, Wen-Hsin and Wang, Sheng-Yang
Journal: Plants (2024): 321
Authors: Hsiao, Wen-Wei and Lau, Ka-Man and Chien, Shih-Chang and Chu, Fang-Hua and Chung, Wen-Hsin and Wang, Sheng-Yang
Journal: Plants (2024): 321
Pterostilbene-Isothiocyanate Inhibits Proliferation of Human MG-63 Osteosarcoma Cells via Abrogating $\beta$-Catenin/TCF-4 Interaction─ A Mechanistic Insight
Authors: Kumar, Viney and Haldar, Swati and Ghosh, Souvik and Saini, Saakshi and Dhankhar, Poonam and Roy, Partha
Journal: ACS Omega (2023)
Authors: Kumar, Viney and Haldar, Swati and Ghosh, Souvik and Saini, Saakshi and Dhankhar, Poonam and Roy, Partha
Journal: ACS Omega (2023)
Antiapoptotic and antioxidant effects of melatonin on cat vitrified oocytes
Authors: Colombo, M and Mascaro, A and Pecile, A and Fusi, J and Luvoni, GC and others,
Journal: REPRODUCTION IN DOMESTIC ANIMALS (2023): 192--192
Authors: Colombo, M and Mascaro, A and Pecile, A and Fusi, J and Luvoni, GC and others,
Journal: REPRODUCTION IN DOMESTIC ANIMALS (2023): 192--192
References
View all 21 references: Citation Explorer
Vitex Rotundifolia Fractions Induced Apoptosis in Human Breast Cancer T-47D Cell Line via Activation of Extrinsic and Intrinsic Pathway.
Authors: Chaudhry, Gul-E-Saba and Jan, Rehmat and Naveed Zafar, Muhammad and Mohammad, Habsah and Muhammad, Tengku Sifzizul Tengku
Journal: Asian Pacific journal of cancer prevention : APJCP (2019): 3555-3562
Authors: Chaudhry, Gul-E-Saba and Jan, Rehmat and Naveed Zafar, Muhammad and Mohammad, Habsah and Muhammad, Tengku Sifzizul Tengku
Journal: Asian Pacific journal of cancer prevention : APJCP (2019): 3555-3562
A mechanism for semaphorin-induced apoptosis: DNA damage of endothelial and myogenic cells in primary cultures from skeletal muscle.
Authors: Hei Yuan, Haynes Shek and Katyal, Sachin and Anderson, Judy E
Journal: Oncotarget (2018): 22618-22630
Authors: Hei Yuan, Haynes Shek and Katyal, Sachin and Anderson, Judy E
Journal: Oncotarget (2018): 22618-22630
MyD88 mediates the decision to die by apoptosis or necroptosis after UV irradiation.
Authors: Harberts, Erin and Fishelevich, Rita and Liu, Juan and Atamas, Sergei P and Gaspari, Anthony A
Journal: Innate immunity (2014): 529-39
Authors: Harberts, Erin and Fishelevich, Rita and Liu, Juan and Atamas, Sergei P and Gaspari, Anthony A
Journal: Innate immunity (2014): 529-39
Damaging legacy: maternal cigarette smoking has long-term consequences for male offspring fertility.
Authors: Sobinoff, A P and Sutherland, J M and Beckett, E L and Stanger, S J and Johnson, R and Jarnicki, A G and McCluskey, A and St John, J C and Hansbro, P M and McLaughlin, E A
Journal: Human reproduction (Oxford, England) (2014): 2719-35
Authors: Sobinoff, A P and Sutherland, J M and Beckett, E L and Stanger, S J and Johnson, R and Jarnicki, A G and McCluskey, A and St John, J C and Hansbro, P M and McLaughlin, E A
Journal: Human reproduction (Oxford, England) (2014): 2719-35
α-Lipoic acid attenuates light insults to neurones.
Authors: Ji, Dan and Majid, Aman Shah Abdul and Yin, Zheng Qin
Journal: Biological & pharmaceutical bulletin (2013): 1060-7
Authors: Ji, Dan and Majid, Aman Shah Abdul and Yin, Zheng Qin
Journal: Biological & pharmaceutical bulletin (2013): 1060-7
Page updated on October 8, 2024