Tide Fluor™ 8WS alkyne
TF8WS alkyne; Near Infrared Emission
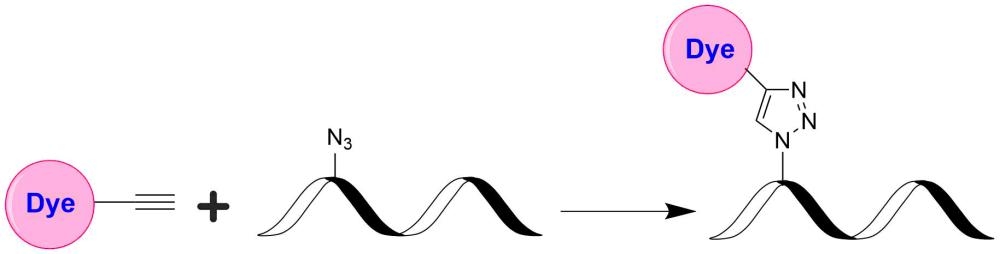
Tide Fluor™ 8WS (TF8WS) family has the spectral properties similar to those of IRDye 800. Their fluorescence is pH-independent from pH 3 to 11. These characteristics make this new dye family more robust to pH-sensitive assays. In some cases TF7-labeled peptides and nucleotides exhibit stronger fluorescence and higher photostability than the ones labeled with IRDye 800. In pairing with our Tide Quencher™ 8WS (TQ8WS), a variety of FRET peptides and nucleotides can be developed for detecting proteases and molecular beacons with enhanced sensitivity and stability. This TF8WS product is reactive to azides, and useful for click chemistry.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2307 | 1 mg | Price |
Physical properties
| Molecular weight | 1094.18 |
| Solvent | DMSO |
Spectral properties
| Correction factor (280 nm) | 0.109 |
| Extinction coefficient (cm -1 M -1) | 250000 |
| Excitation (nm) | 785 |
| Emission (nm) | 801 |
| Quantum yield | 0.21 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 18, 2026

