ReadiLink™ xtra Rapid iFluor® 488 Antibody Labeling Kit *BSA-Compatible*
ReadiLink™ xtra rapid antibody labeling kits require essentially only 2 simple mixing steps without a column purification needed. Preactivated iFluor® 488 used in this ReadiLink™ kit is quite stable and shows good reactivity and selectivity with antibodies. The kit has all the essential components for labeling ~2x50 ug antibody. Each of the two vials of preactivated iFluor® 488 dye provided in the kit is optimized for labeling ~50 µg antibody. ReadiLink™ xtra iFluor® 488 rapid antibody labeling kit provides a convenient and robust method to label monoclonal and polyclonal antibodies with the bright green fluorescent iFluor® 488 fluorophore. AAT Bioquest's iFluor® dyes are optimized for labeling proteins, in particular, antibodies. These dyes are bright, photostable and have minimal quenching on proteins. They can be well excited by the major laser lines of fluorescence instruments (e.g., 350, 405, 488, 555 and 633 nm). 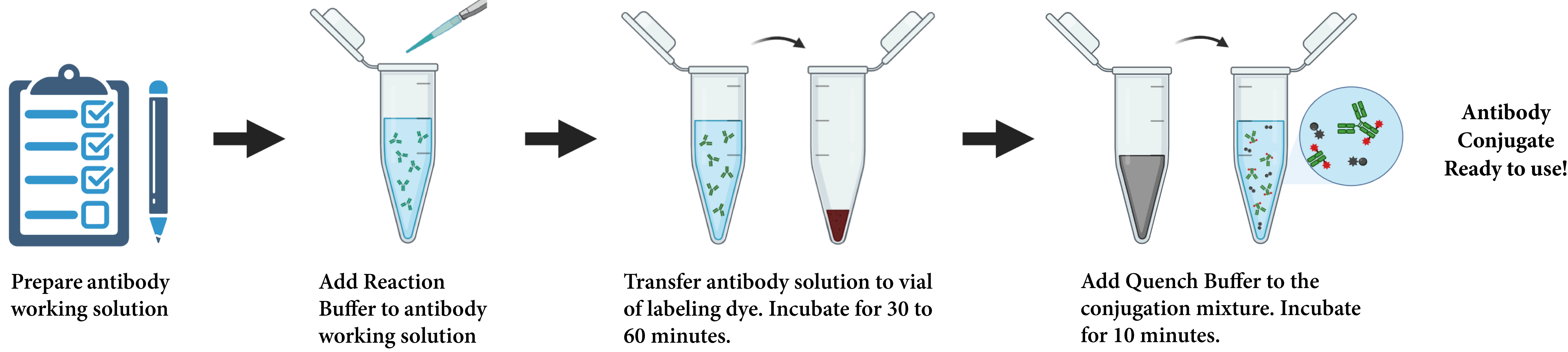

Figure 1. Overview of the ReadiLink™ xtra Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.
Example protocol
AT A GLANCE
Important
Warm all the components and centrifuge the vials briefly before opening, and immediately prepare the required solutions before starting your conjugation. The following protocol is for recommendation.PREPARATION OF WORKING SOLUTION
Protein working solution (Solution A)
For labeling 50 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 5 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 50 µL of the target protein solution.Note If you have a different protein concentration, adjust the protein volume accordingly to make ~50 µg of protein available for your labeling reaction.
Note For labeling 100 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 10 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 100 µL of the target protein solution.
Note The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2 - 7.4; if the protein is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra-0.5, Ultracel-10 Membrane, 10 kDa (cat# UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) with 0.1 to 0.5 % will be labeled well.
Note For optimal labeling efficiency, a final protein concentration range of 1 - 2 mg/mL is recommended, with a significantly reduced conjugation efficiency at less than 1 mg/mL.
SAMPLE EXPERIMENTAL PROTOCOL
Run conjugation reaction
- Add the protein working solution (Solution A) to ONE vial of labeling dye (Component A), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Note If labeling 100 µg of protein, use both vials (Component A) of labeling dye by dividing the 100 µg of protein into 2 x 50 µg of protein and reacting each 50 µg of protein with one vial of labeling dye. Then combine both vials for the next step. - Keep the conjugation reaction mixture at room temperature for 30 - 60 minutes.
Note The conjugation reaction mixture can be rotated or shaken for longer time if desired.
Stop Conjugation reaction
- Add 5 µL (for 50 µg protein) or 10 µL (for 100 µg protein) which is 10% of the total reaction volume of TQ™-Dyed Quench Buffer (Component C) into the conjugation reaction mixture; mix well.
- Incubate at room temperature for 10 minutes. The labeled protein (antibody) is now ready to use.
Storage of Protein Conjugate
The protein conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). For longer storage, the protein conjugates could be lyophilized or divided into single-used aliquots and stored at ≤ –20 °C.Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| ReadiLink™ xtra Rapid iFluor® 350 Antibody Labeling Kit *BSA-Compatible* | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| ReadiLink™ xtra Rapid iFluor® 555 Antibody Labeling Kit *BSA-Compatible* | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| ReadiLink™ xtra Rapid iFluor® 594 Antibody Labeling Kit *BSA-Compatible* | 587 | 603 | 2000001 | 0.531 | 0.05 | 0.04 |
| ReadiLink™ xtra Rapid iFluor® 647 Antibody Labeling Kit *BSA-Compatible* | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| ReadiLink™ xtra Rapid iFluor® 750 Antibody Labeling Kit *BSA-Compatible* | 757 | 779 | 2750001 | 0.121 | 0.044 | 0.039 |
References
View all 27 references: Citation Explorer
Author Correction: A dynamic three-step mechanism drives the HIV-1 pre-fusion reaction.
Authors: Iliopoulou, Maro and Nolan, Rory and Alvarez, Luis and Watanabe, Yasunori and Coomer, Charles A and Jakobsdottir, G Maria and Bowden, Thomas A and Padilla-Parra, Sergi
Journal: Nature structural & molecular biology (2019): 526
Authors: Iliopoulou, Maro and Nolan, Rory and Alvarez, Luis and Watanabe, Yasunori and Coomer, Charles A and Jakobsdottir, G Maria and Bowden, Thomas A and Padilla-Parra, Sergi
Journal: Nature structural & molecular biology (2019): 526
Intracellular in situ labeling of TiO2 nanoparticles for fluorescence microscopy detection.
Authors: Brown, Koshonna and Thurn, Ted and Xin, Lun and Liu, William and Bazak, Remon and Chen, Si and Lai, Barry and Vogt, Stefan and Jacobsen, Chris and Paunesku, Tatjana and Woloschak, Gayle E
Journal: Nano research (2018): 464-476
Authors: Brown, Koshonna and Thurn, Ted and Xin, Lun and Liu, William and Bazak, Remon and Chen, Si and Lai, Barry and Vogt, Stefan and Jacobsen, Chris and Paunesku, Tatjana and Woloschak, Gayle E
Journal: Nano research (2018): 464-476
Effect of Fluorescent Labels on Peptide and Amino Acid Sample Dimensionality in Two Dimensional nLC × μFFE Separations.
Authors: Geiger, Matthew and Bowser, Michael T
Journal: Analytical chemistry (2016): 2177-87
Authors: Geiger, Matthew and Bowser, Michael T
Journal: Analytical chemistry (2016): 2177-87
Probing minority population of antibiotic-resistant bacteria.
Authors: Huang, Tianxun and Zheng, Yan and Yan, Ya and Yang, Lingling and Yao, Yihui and Zheng, Jiaxin and Wu, Lina and Wang, Xu and Chen, Yuqing and Xing, Jinchun and Yan, Xiaomei
Journal: Biosensors & bioelectronics (2016): 323-330
Authors: Huang, Tianxun and Zheng, Yan and Yan, Ya and Yang, Lingling and Yao, Yihui and Zheng, Jiaxin and Wu, Lina and Wang, Xu and Chen, Yuqing and Xing, Jinchun and Yan, Xiaomei
Journal: Biosensors & bioelectronics (2016): 323-330
Label-free imaging of gelatin-containing hydrogel scaffolds.
Authors: Liang, Yajie and Bar-Shir, Amnon and Song, Xiaolei and Gilad, Assaf A and Walczak, Piotr and Bulte, Jeff W M
Journal: Biomaterials (2015): 144-50
Authors: Liang, Yajie and Bar-Shir, Amnon and Song, Xiaolei and Gilad, Assaf A and Walczak, Piotr and Bulte, Jeff W M
Journal: Biomaterials (2015): 144-50
Page updated on March 11, 2025





