ReadiLink™ xtra Rapid iFluor® 350 Antibody Labeling Kit *BSA-Compatible*
ReadiLink™ xtra rapid antibody labeling kits require essentially only 2 simple mixing steps without a column purification needed. Preactivated iFluor® 350 used in this ReadiLink™ kit is quite stable and shows good reactivity and selectivity with antibodies. The kit has all the essential components for labeling ~2x50 ug antibody. Each of the two vials of preactivated iFluor® 350 dye provided in the kit is optimized for labeling ~50 µg antibody. ReadiLink™ xtra iFluor® 350 rapid antibody labeling kit provides a convenient and robust method to label monoclonal and polyclonal antibodies with the bright blue fluorescent iFluor® 350 fluorophore. AAT Bioquest's iFluor® dyes are optimized for labeling proteins, particularly antibodies. These dyes are bright, photostable, and have minimal quenching on proteins. They can be well excited by the major laser lines of fluorescence instruments (e.g., 350, 405, 488, 555, and 633 nm). 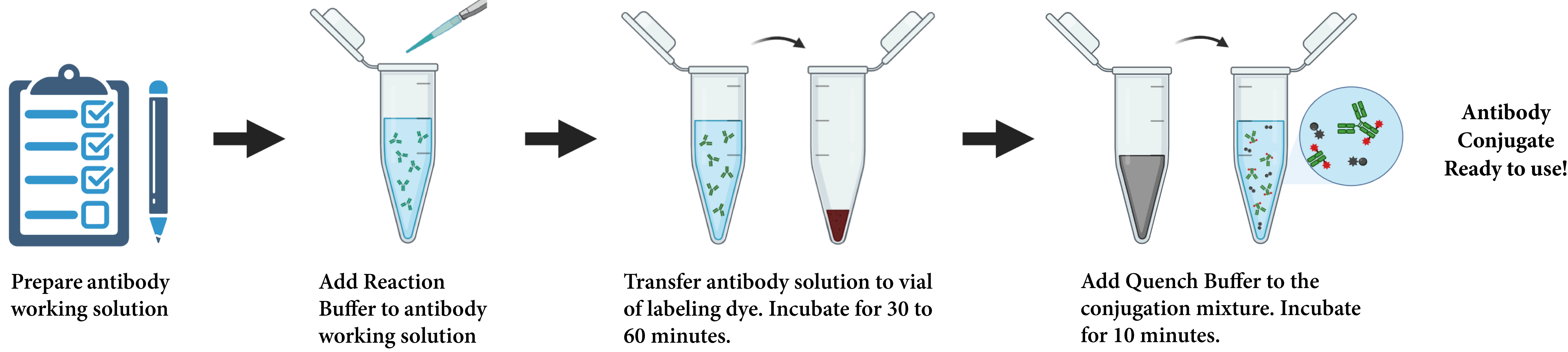

Figure 1. Overview of the ReadiLink™ xtra Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.
Example protocol
AT A GLANCE
Important
Warm all the components and centrifuge the vials briefly before opening, and immediately prepare the required solutions before starting your conjugation. The following protocol is for recommendation.PREPARATION OF WORKING SOLUTION
Protein working solution (Solution A)
For labeling 50 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 5 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 50 µL of the target protein solution.Note If you have a different protein concentration, adjust the protein volume accordingly to make ~50 µg of protein available for your labeling reaction.
Note For labeling 100 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 10 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 100 µL of the target protein solution.
Note The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2 - 7.4; if the protein is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra-0.5, Ultracel-10 Membrane, 10 kDa (cat# UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) with 0.1 to 0.5 % will be labeled well.
Note For optimal labeling efficiency, a final protein concentration range of 1 - 2 mg/mL is recommended, with a significantly reduced conjugation efficiency at less than 1 mg/mL.
SAMPLE EXPERIMENTAL PROTOCOL
Run conjugation reaction
- Add the protein working solution (Solution A) to ONE vial of labeling dye (Component A), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Note If labeling 100 µg of protein, use both vials (Component A) of labeling dye by dividing the 100 µg of protein into 2 x 50 µg of protein and reacting each 50 µg of protein with one vial of labeling dye. Then combine both vials for the next step. - Keep the conjugation reaction mixture at room temperature for 30 - 60 minutes.
Note The conjugation reaction mixture can be rotated or shaken for longer time if desired.
Stop Conjugation reaction
- Add 5 µL (for 50 µg protein) or 10 µL (for 100 µg protein) which is 10% of the total reaction volume of TQ™-Dyed Quench Buffer (Component C) into the conjugation reaction mixture; mix well.
- Incubate at room temperature for 10 minutes. The labeled protein (antibody) is now ready to use.
Storage of Protein Conjugate
The protein conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). For longer storage, the protein conjugates could be lyophilized or divided into single-used aliquots and stored at ≤ –20°C.Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| ReadiLink™ xtra Rapid iFluor® 488 Antibody Labeling Kit *BSA-Compatible* | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| ReadiLink™ xtra Rapid iFluor® 555 Antibody Labeling Kit *BSA-Compatible* | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| ReadiLink™ xtra Rapid iFluor® 594 Antibody Labeling Kit *BSA-Compatible* | 587 | 603 | 2000001 | 0.531 | 0.05 | 0.04 |
| ReadiLink™ xtra Rapid iFluor® 647 Antibody Labeling Kit *BSA-Compatible* | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| ReadiLink™ xtra Rapid iFluor® 750 Antibody Labeling Kit *BSA-Compatible* | 757 | 779 | 2750001 | 0.121 | 0.044 | 0.039 |
References
View all 93 references: Citation Explorer
Identification of a Small Probe That Can Be Conjugated to Proteins by Proximity Labeling.
Authors: Sun, Weiping and Huo, Yinbo and Mei, Yuxuan and Zhou, Qingtong and Zhao, Suwen and Zhuang, Min
Journal: ACS chemical biology (2020): 39-43
Authors: Sun, Weiping and Huo, Yinbo and Mei, Yuxuan and Zhou, Qingtong and Zhao, Suwen and Zhuang, Min
Journal: ACS chemical biology (2020): 39-43
Paper-based nuclease protection assay with on-chip sample pretreatment for point-of-need nucleic acid detection.
Authors: Noviana, Eka and Jain, Sidhartha and Hofstetter, Josephine and Geiss, Brian J and Dandy, David S and Henry, Charles S
Journal: Analytical and bioanalytical chemistry (2020): 3051-3061
Authors: Noviana, Eka and Jain, Sidhartha and Hofstetter, Josephine and Geiss, Brian J and Dandy, David S and Henry, Charles S
Journal: Analytical and bioanalytical chemistry (2020): 3051-3061
Site-Specific Fluorescent Labeling of Antibodies and Diabodies Using SpyTag/SpyCatcher System for In Vivo Optical Imaging.
Authors: Alam, Md Kausar and El-Sayed, Ayman and Barreto, Kris and Bernhard, Wendy and Fonge, Humphrey and Geyer, C Ronald
Journal: Molecular imaging and biology (2019): 54-66
Authors: Alam, Md Kausar and El-Sayed, Ayman and Barreto, Kris and Bernhard, Wendy and Fonge, Humphrey and Geyer, C Ronald
Journal: Molecular imaging and biology (2019): 54-66
A neuraminidase potency assay for quantitative assessment of neuraminidase in influenza vaccines.
Authors: Byrne-Nash, Rose T and Gillis, Jacob H and Miller, David F and Bueter, Katie M and Kuck, Laura R and Rowlen, Kathy L
Journal: NPJ vaccines (2019): 3
Authors: Byrne-Nash, Rose T and Gillis, Jacob H and Miller, David F and Bueter, Katie M and Kuck, Laura R and Rowlen, Kathy L
Journal: NPJ vaccines (2019): 3
Author Correction: A dynamic three-step mechanism drives the HIV-1 pre-fusion reaction.
Authors: Iliopoulou, Maro and Nolan, Rory and Alvarez, Luis and Watanabe, Yasunori and Coomer, Charles A and Jakobsdottir, G Maria and Bowden, Thomas A and Padilla-Parra, Sergi
Journal: Nature structural & molecular biology (2019): 526
Authors: Iliopoulou, Maro and Nolan, Rory and Alvarez, Luis and Watanabe, Yasunori and Coomer, Charles A and Jakobsdottir, G Maria and Bowden, Thomas A and Padilla-Parra, Sergi
Journal: Nature structural & molecular biology (2019): 526
Page updated on April 15, 2025



