ReadiLink™ Rapid Cy7 Antibody Labeling Kit *Microscale Optimized for Labeling 50 μg Antibody Per Reaction*
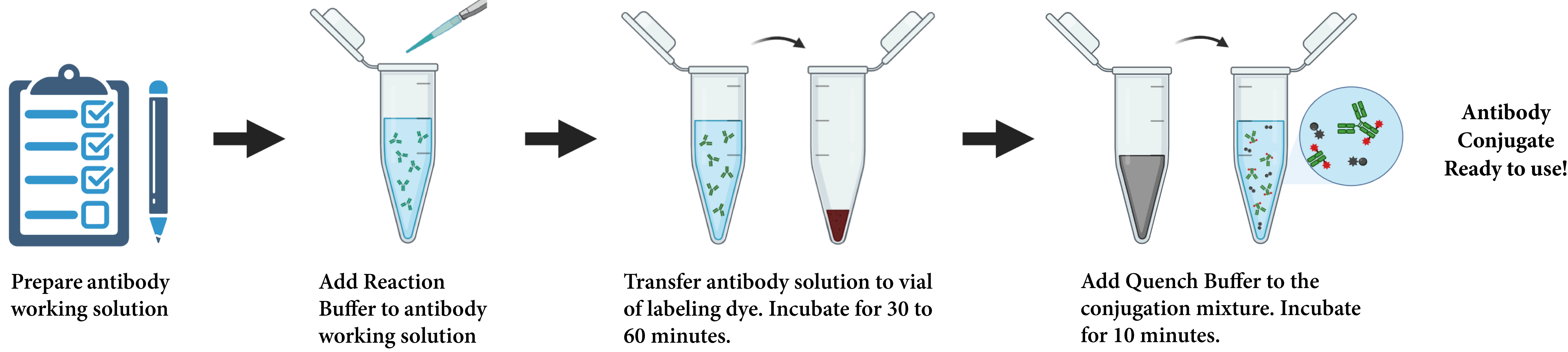
Figure 1. Overview of the ReadiLink™ Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.
Example protocol
AT A GLANCE
Warm all the components and centrifuge the vials briefly before opening, and immediately prepare the required solutions before starting your conjugation. The following protocol is for recommendation.
PREPARATION OF WORKING SOLUTION
For labeling 50 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 5 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 50 µL of the target protein solution.
Note: If you have a different protein concentration, adjust the protein volume accordingly to make ~50 µg of protein available for your labeling reaction.
Note: For labeling 100 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 10 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 100 µL of the target protein solution.
Note: The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2 - 7.4; if the protein is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra-0.5, Ultracel-10 Membrane, 10 kDa (cat# UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
Note: For optimal labeling efficiency, a final protein concentration range of 1 - 2 mg/mL is recommended, with a significantly reduced conjugation efficiency at less than 1 mg/mL.
SAMPLE EXPERIMENTAL PROTOCOL
Add the protein working solution (Solution A) to ONE vial of labeling dye (Component A), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Note: If labeling 100 µg of protein, use both vials (Component A) of labeling dye by dividing the 100 µg of protein into 2 x 50 µg of protein and reacting each 50 µg of protein with one vial of labeling dye. Then combine both vials for the next step.
Keep the conjugation reaction mixture at room temperature for 30 - 60 minutes.
Note: The conjugation reaction mixture can be rotated or shaken for longer time if desired.
- Add 5 µL (for 50 µg protein) or 10 µL (for 100 µg protein) which is 10% of the total reaction volume of TQ™-Dyed Quench Buffer (Component C) into the conjugation reaction mixture; mix well.
- Incubate at room temperature for 10 minutes. The labeled protein (antibody) is now ready to use.
The protein conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). For longer storage, the protein conjugates could be lyophilized or divided into single-used aliquots and stored at ≤ –20°C.
Spectrum
Product family
Citations
Authors: Wang, Xiaoyan and Zhang, Yu and Xue, Wei and Wang, Hong and Qiu, Xiaozhong and Liu, Zonghua
Journal: Journal of Biomaterials Applications (2017): 923--932
Authors: Guo, Fuqiang and Shang, Jiajia and Zhao, Hai and Lai, Kangrong and Li, Yang and Fan, Zhongxiong and Hou, Zhenqing and Su, Guanghao
Journal: Colloids and Surfaces B: Biointerfaces (2017)
Authors: Guo, Yuxin and Zhang, Yang and Ma, Jinyuan and Li, Qi and Li, Yang and Zhou, Xinyi and Zhao, Dan and Song, Hua and Chen, Qing and Zhu, Xuan
Journal: Journal of Controlled Release (2017)
Authors: Deng, Xiongwei and Yin, Zhaoxia and Zhou, Zhixiang and Wang, Yihui and Zhang, Fang and Hu, Qin and Yang, Yishu and Lu, Jianqing and Wu, Yan and Sheng, Wang and others, undefined
Journal: ACS applied materials & interfaces (2016): 17068--17079
Authors: Wang, Xu and Shi, Changying and Zhang, Li and Bodman, Alexa and Guo, D and an , undefined and Wang, Lili and Hall, Walter A and Wilkens, Stephan and Luo, Juntao
Journal: Biomaterials (2016)
References
Authors: Freeman LM, Li S, Dayani Y, Choi HS, Malmstadt N, Armani AM.
Journal: Appl Phys Lett (2011): 143703
Authors: Wiessler M, Hennrich U, Pipkorn R, Waldeck W, Cao L, Peter J, Ehemann V, Semmler W, Lammers T, Braun K.
Journal: Theranostics (2011): 381
Authors: Berezin MY, Guo K, Akers W, Livingston J, Solomon M, Lee H, Liang K, Agee A, Achilefu S.
Journal: Biochemistry (2011): 2691
Authors: Adler ED, Bystrup A, Briley-Saebo KC, Mani V, Young W, Giovanonne S, Altman P, Kattman SJ, Frank JA, Weinmann HJ, Keller GM, Fayad ZA.
Journal: JACC Cardiovasc Imaging (2009): 1114
Authors: Volke D, Hoffmann R.
Journal: Electrophoresis (2008): 4516



