Cal-590™ AM
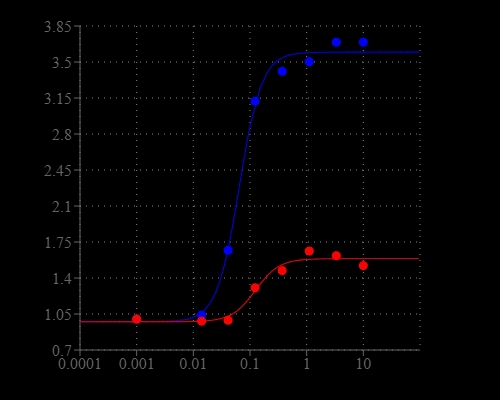
Calcium measurement is critical for numerous biological investigations. Fluorescent probes that show spectral responses upon binding calcium have enabled researchers to investigate changes in intracellular free calcium concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. Rhod-2 is most commonly used among the red fluorescent calcium indicators. However, Rhod-2 AM is only moderately fluorescent in live cells upon esterase hydrolysis, and has very small cellular calcium responses. Cal-590™ has been developed to improve Rhod-2 cell loading and calcium response while maintaining the spectral wavelength of Rhod-2, making it compatible with TRITC/Cy3® filter set. In CHO and HEK cells Cal-590™ AM has cellular calcium response that is much more sensitive than Rhod-2 AM. The spectra of Cal-590 is well separated from those of FITC, Alexa Fluor® 488 and GFP, making it an ideal calcium probe for multiplexing intracellular assays with GFP cell lines or FITC/Alexa Fluor® 488 labeled antibodies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20510 | 5x50 ug | Price | |
| 20511 | 10x50 ug | Price | |
| 20512 | 1 mg | Price |
Physical properties
| Dissociation constant (Kd, nM) | 561 |
| Molecular weight | 1266.81 |
| Solvent | DMSO |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 78000 |
| Excitation (nm) | 574 |
| Emission (nm) | 588 |
| Quantum yield | 0.62 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | TRITC/Cy3 |
| Emission | TRITC/Cy3 |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 540 |
| Emission | 590 |
| Cutoff | 570 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 15, 2026

