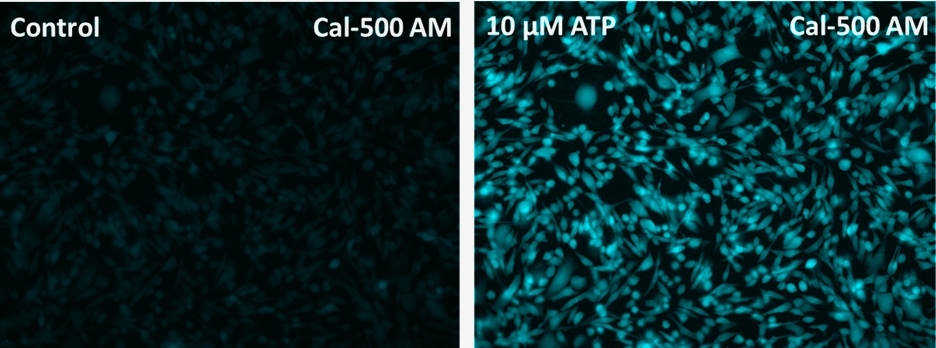
Cal-500™ AM
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 2 to 5 mM stock solution of Cal-500™ AM in anhydrous DMSO.
Note: When reconstituted in DMSO, Cal-500™ AM is a clear, colorless solution.
PREPARATION OF WORKING SOLUTION
On the day of the experiment, either dissolve Cal-500™ AM in DMSO or thaw an aliquot of the indicator stock solution to room temperature.
Prepare a 2 to 20 µM Cal-500™ AM working solution in a buffer of your choice (e.g., Hanks and Hepes buffer) with 0.04% Pluronic® F-127. For most cell lines, Cal-500™ AM at a final concentration of 4-5 μM is recommended. The exact concentration of indicators required for cell loading must be determined empirically.
Note: The nonionic detergent Pluronic® F-127 is sometimes used to increase the aqueous solubility of Cal-500™ AM. A variety of Pluronic® F-127 solutions can be purchased from AAT Bioquest.
Note: If your cells contain organic anion-transporters, probenecid (1-2 mM) may be added to the dye working solution (final in well concentration will be 0.5-1 mM) to reduce leakage of the de-esterified indicators. A variety of ReadiUse™ Probenecid products, including water-soluble, sodium salt, and stabilized solutions, can be purchased from AAT Bioquest.
SAMPLE EXPERIMENTAL PROTOCOL
Following is our recommended protocol for loading AM esters into live cells. This protocol only provides a guideline and should be modified according to your specific needs.
- Prepare cells in growth medium overnight.
On the next day, add 1X Cal-500™ AM working solution to your cell plate.
Note: If your compound(s) interfere with the serum, replace the growth medium with fresh HHBS buffer before dye-loading.
Incubate the dye-loaded plate in a cell incubator at 37 °C for 30 to 60 minutes.
Note: Incubating the dye for longer than 2 hours can improve signal intensities in certain cell lines.
- Replace the dye working solution with HHBS or buffer of your choice (containing an anion transporter inhibitor, such as 1 mM probenecid, if applicable) to remove any excess probes.
- Add the stimulant as desired and simultaneously measure fluorescence using either a fluorescence microscope equipped with a DAPI filter set or a fluorescence plate reader containing a programmable liquid handling system such as an FDSS, FLIPR, or FlexStation, at Ex/Em = 400/500 nm cutoff 470 nm.
Calculators
Common stock solution preparation
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 98.047 µL | 490.235 µL | 980.469 µL | 4.902 mL | 9.805 mL |
| 5 mM | 19.609 µL | 98.047 µL | 196.094 µL | 980.469 µL | 1.961 mL |
| 10 mM | 9.805 µL | 49.023 µL | 98.047 µL | 490.235 µL | 980.469 µL |
Molarity calculator
| Mass (Calculate) | Molecular weight | Volume (Calculate) | Concentration (Calculate) | Moles | ||||
| / | = | x | = |
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Quantum yield |
| Cal-520®, AM | 492 | 515 | 0.751 |
| Cal-520FF™, AM | 492 | 515 | 0.751 |
| Cal-520N™, AM | 492 | 515 | 0.751 |
| Cal-590™ AM | 574 | 588 | 0.621 |
| Cal-630™ AM | 609 | 626 | 0.371 |
Citations
Authors: Wu, Yanjiao and Xu, Xiaoli and Ma, Lunkun and Yi, Qian and Sun, Weichao and Tang, Liling
Journal: The International Journal of Biochemistry & Cell Biology (2017)
Authors: Lu, Jiang and Yao, Xue-qin and Luo, Xin and Wang, Yu and Chung, Sookja Kim and Tang, He-xin and Cheung, Chi Wai and Wang, Xian-yu and Meng, Chen and Li, Qing and others, undefined
Journal: Neural Regeneration Research (2017): 945
Authors: Yang, Gang and Xiao, Zhenghua and Ren, Xiaomei and Long, Haiyan and Ma, Kunlong and Qian, Hong and Guo, Yingqiang
Journal: Scientific Reports (2017): 41781
Authors: Liu, Jia and Du, Qing and Zhu, He and Li, Yu and Liu, Maodong and Yu, Shoushui and Wang, Shilei
Journal: Int J Clin Exp Med (2017): 6861--6868
Authors: Sun, Xia and Lin, Yi and Huang, Qiansheng and Shi, Junpeng and Qiu, Ling and Kang, Mei and Chen, Yajie and Fang, Chao and Ye, Ting and Dong, Sijun
Journal: Journal of cellular and molecular medicine (2015): 581--594
References
Authors: Bailey S, Macardle PJ.
Journal: J Immunol Methods (2006): 220
Authors: Orlicky J, Sulova Z, Dovinova I, Fiala R, Zahradnikova A, Jr., Breier A.
Journal: Gen Physiol Biophys (2004): 357
Authors: Patel H, Porter RH, Palmer AM, Croucher MJ.
Journal: Br J Pharmacol (2003): 671
Authors: Loughrey CM, MacEachern KE, Cooper J, Smith GL.
Journal: Cell Calcium (2003): 1
Authors: Rockwell PL, Storey BT.
Journal: Mol Reprod Dev (2000): 335