trFluor™ Eu Acceptor XL665
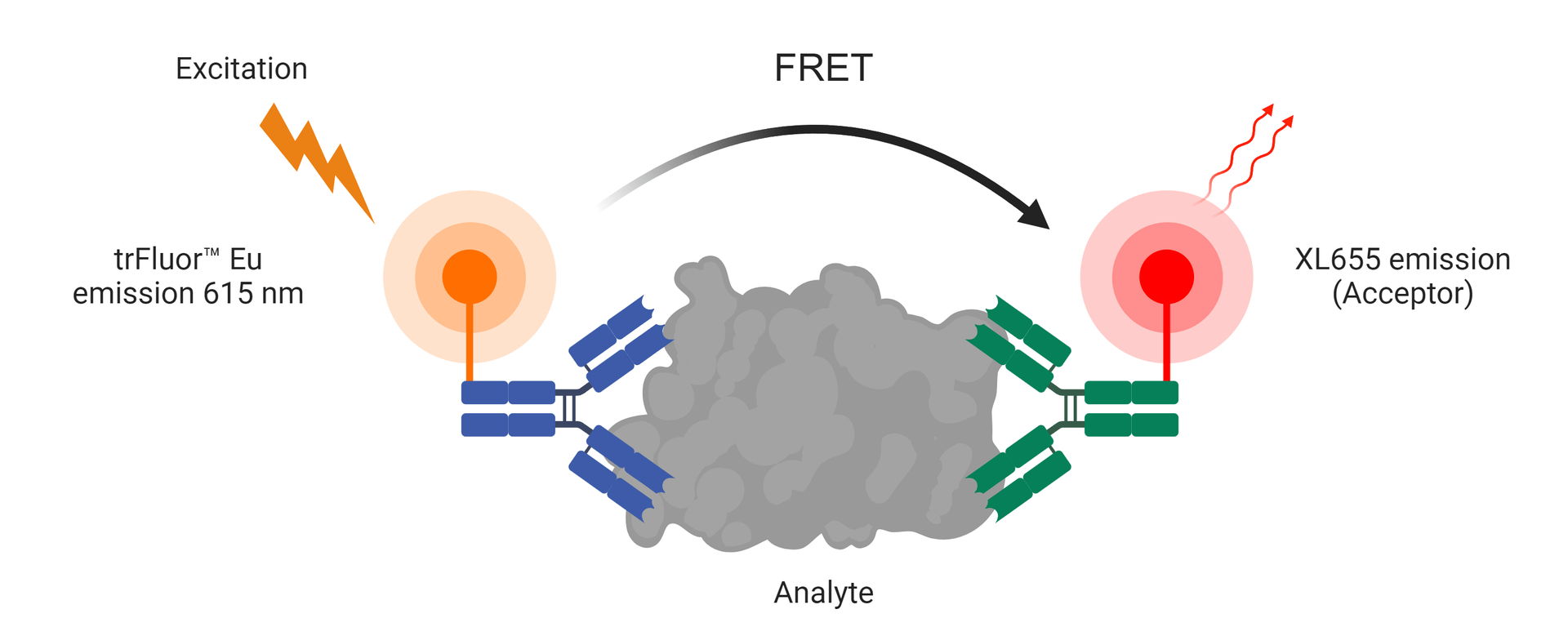
trFluor™ Eu Acceptor XL665 is an excellent replacement for the commonly used D2 acceptor in labeling antibodies or antigens for bioconjugates paired with europium (Eu)-labeled probes in TR-FRET assays. Eu probes enable time-resolved fluorometry (TRF) in assays requiring high sensitivity. They exhibit large Stokes Shifts and exceptionally long emission half-lives compared to traditional fluorophores such as Alexa Fluor or cyanine dyes. In comparison to other TRF compounds, our TR Fluor™ Eu probes offer high stability, high emission yield, and the ability to be linked to various biomolecules. These probes serve as excellent donors for developing TR-FRET assays in conjunction with trFluor™ Eu Acceptor XL665. Since many biological compounds found in cells, serum, or other biological fluids are naturally fluorescent, using conventional, prompt fluorophores can significantly limit assay sensitivity due to the high background caused by the autofluorescence of the biological molecules to be assayed. Utilizing long-lived fluorophores combined with time-resolved detection, which introduces a delay between excitation and emission detection, minimizes prompt fluorescence interferences.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1440 | 1 mg | Price |
Physical properties
| Molecular weight | ~105000 |
| Solvent | Water (Add 100 µL of H₂O to Cat# 2503 or 1 mL to Cat# 2504 to reconstitute to 10 mg/mL in PBS) |
Spectral properties
| Excitation (nm) | 652 |
| Emission (nm) | 659 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 28, 2026

