Thioflavin T
Thioflavin T is a cell-permeable fluorescent dye that binds to amyloid fibrils, widely used for detecting protein aggregation in Alzheimer's disease and related research.
- Amyloid-Specific Binding: >1000-fold fluorescence increase upon β-sheet interaction
- Enhanced Quantum Yield: QY increases from 0.0001 to 0.43 when bound to fibrils
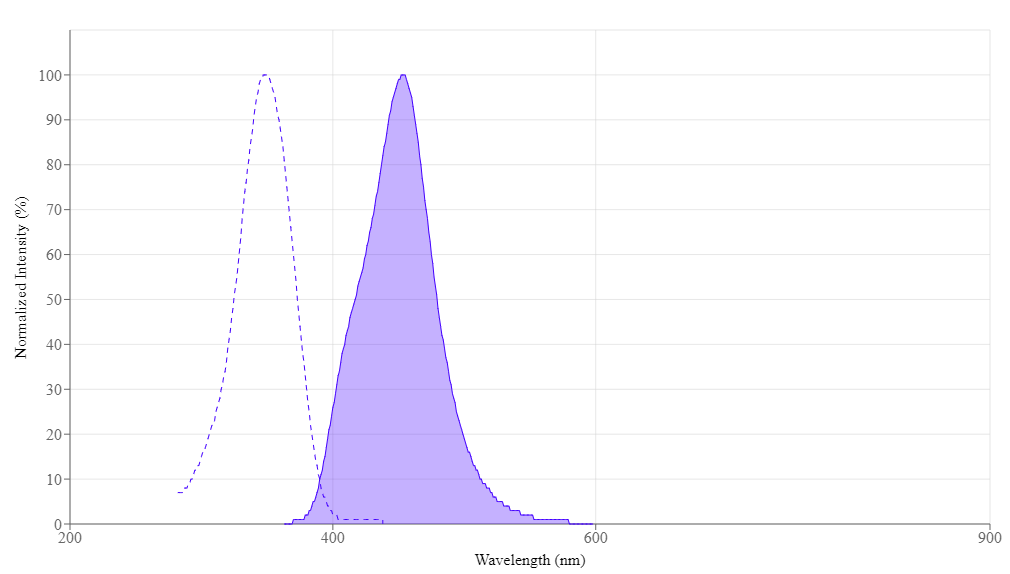
- Excitation/Emission Shift: Free dye (350/438 nm) shifts to 450/482 nm when bound
- Rapid Binding Kinetics: Real-time monitoring of amyloid formation

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 23060 | 1 g | Price |
Physical properties
| Molecular weight | 318.87 |
| Solvent | DMSO |
Spectral properties
| Absorbance (nm) | 199 |
| Excitation (nm) | 349 |
| Emission (nm) | 454 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 2390-54-7 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 22, 2026

