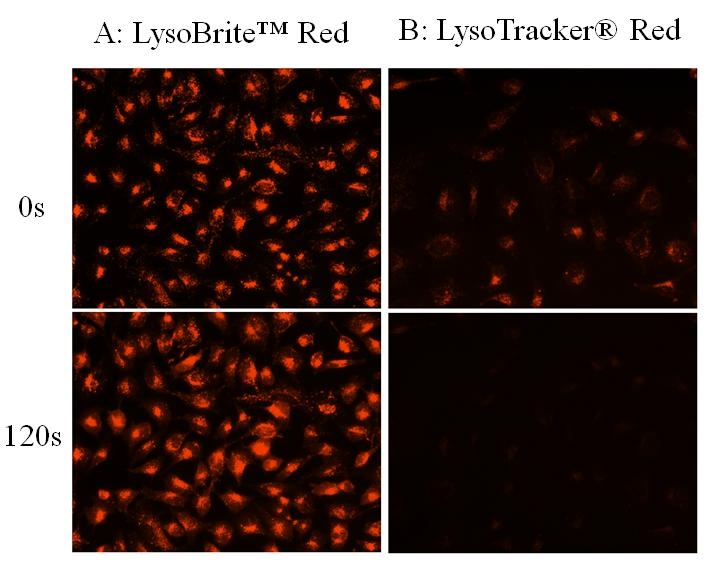
LysoBrite™ Red
Example protocol
AT A GLANCE
Prepare cells.
Add dye working solution.
Incubate at 37 °C for 30 minutes.
Wash the cells.
Analyze under a fluorescence microscope.
The LysoBrite™ Red stock solution provided is 500X in DMSO. It should be stable for at least 6 months if stored at -20°C and protected from light. Avoid freeze/thaw cycles.
PREPARATION OF WORKING SOLUTION
Warm LysoBrite™ Red dye to room temperature.
Prepare dye working solution by diluting 20 µL of 500X LysoBrite™ Red with 10 mL of Hanks and 20 mM HEPES buffer (HBSS) or buffer of your choice.
Note: 20 µL of LysoBrite™ Red dye is enough for one 96-well plate. Aliquot and store unused LysoBrite™ dye stock solutions at < -15 °C. Protect it from light and avoid repeated freeze-thaw cycles.
Note: The optimal concentration of the fluorescent lysosome indicator varies depending on the specific application. The staining conditions may be modified according to the particular cell type and the permeability of the cells or tissues to the probe.
SAMPLE EXPERIMENTAL PROTOCOL
This protocol only provides a guideline and should be modified according to your specific needs.
Grow cells in a 96-well black wall/clear bottom plate (100 µL/well/96-well plate) or on coverslips inside a petri dish filled with the appropriate culture medium.
When cells reach the desired confluence, add an equal volume of the dye-working solution (from Preparation of Working Solution Step 2).
Incubate the cells in a 37 °C, 5% CO2 incubator for 30 minutes.
Wash the cells twice with pre-warmed (37 °C) Hanks and 20 mM HEPES buffer (HBSS) or buffer of your choice. Then fill the cell wells with HBSS or growth medium.
Observe the cells using a fluorescence microscope fitted with the desired filter set.
Note: It is recommended to increase either the labeling concentration or the incubation time to allow the dye to accumulate if the cells do not appear to be sufficiently stained.
Add an equal volume of the dye-working solution (from Preparation of Working Solution Step 2).
Incubate the cells in a 37 °C, 5% CO2 incubator for 30 minutes.
Wash the cells twice with pre-warmed (37 °C) Hanks and 20 mM HEPES buffer (HBSS) or buffer of your choice. Then fill the cell wells with HBSS or growth medium.
Observe the cells using a fluorescence microscope fitted with the desired filter set.
Note: It is recommended to increase either the labeling concentration or the incubation time to allow the dye to accumulate if the cells do not appear to be sufficiently stained.
Note: Suspension cells may be attached to coverslips treated with BD Cell-Tak® (BD Biosciences) and stained as adherent cells (see Protocol for Preparing and Staining Adherent Cells).
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) |
| LysoBrite™ Blue | 434 | 480 |
| LysoBrite™ Green | 501 | 510 |
| LysoBrite™ Orange | 543 | 565 |
| LysoBrite™ Deep Red | 597 | 619 |
| LysoBrite™ NIR | 636 | 651 |
Citations
Authors: Wang, Jue-Rui and Jurado-Aguilar, Javier and Barroso, Emma and Rodr{\'\i}guez-Calvo, Ricardo and Camins, Antoni and Wahli, Walter and Palomer, Xavier and V{\'a}zquez-Carrera, Manuel
Journal: Cell Communication and Signaling (2024): 1--12
Authors: Gaur, Gauri and Predtechenskaya, Maria and Voyich, Jovanka M and James, Garth and Stewart, Philip S and Borgogna, Timothy R
Journal: Microorganisms (2024): 1951
Authors: Han, Yanjie and Lu, Haifeng and Gu, Zikuan and Guan, Peixin and Liu, Zhen
Journal: Nano Today (2024): 102420
Authors: Hirata, Yoko and Takemori, Hiroshi and Furuta, Kyoji and Kamatari, Yuji O and Sawada, Makoto
Journal: Current Research in Pharmacology and Drug Discovery (2024): 100196
Authors: Zhang, Shunyao and Tamura, Atsushi and Yui, Nobuhiko
Journal: Biomolecules (2024): 223
References
Authors: Gough AH, Johnston PA.
Journal: Methods Mol Biol (2007): 41
Authors: Hoffman AF, Garippa RJ.
Journal: Methods Mol Biol (2007): 19
Authors: Giuliano KA., undefined
Journal: Methods Mol Biol (2007): 189
Authors: Taylor DL., undefined
Journal: Methods Mol Biol (2007): 3
Authors: Martinez ED, Dull AB, Beutler JA, Hager GL.
Journal: Methods Enzymol (2006): 21