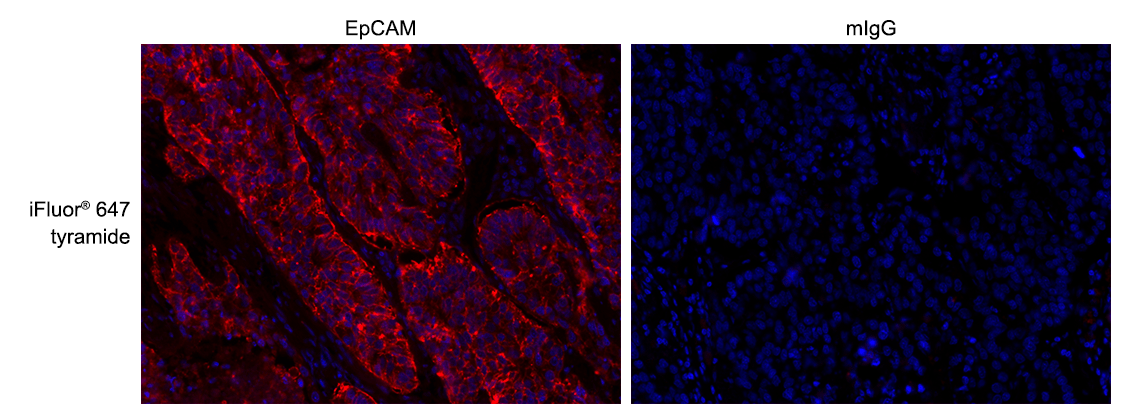
iFluor® 647 Tyramide
For many immunohistochemical (IHC) applications, traditional enzymatic amplification procedures are sufficient for achieving adequate antigen detection. However, several factors limit their sensitivity and utility. Tyramide signal amplification (TSA) has proven to be a particularly versatile and powerful enzyme amplification technique with improved assay sensitivity. TSA is based on the ability of HRP, in the presence of low concentrations of hydrogen peroxide, to convert labeled tyramine-containing substrate into an oxidized, highly reactive free radical that can covalently bind to tyrosine residues at or near the HRP. To achieve maximal IHC detection, tyramine is prelabeled with a fluorophore. The signal amplification conferred by the turnover of multiple tyramide substrates per peroxidase label results in the ability to detect low-abundance targets with ultrasensitive precision and reduces the amount of antibodies and hybridization probes needed. In IHC applications, this method can also enhance sensitivity in cases where the primary antibody dilution needs to be increased to reduce nonspecific background signals or overcome weak immunolabeling due to suboptimal fixation procedures or low levels of target expression. The iFluor® 647 tyramide contains the bright iFluor® 647 that can be readily detected with the standard Cy5 filter set. It is an excellent replacement for Alexa Fluor® 647 tyramide (Alexa Fluor® is the trademark of ThermoFisher), Cy5 tyramide, or other comparable fluorescent tyramide conjugates.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 45110 | 200 Slides | Price |
Physical properties
| Molecular weight | 1092.20 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.03 |
| Correction factor (280 nm) | 0.03 |
| Correction factor (656 nm) | 0.0793 |
| Extinction coefficient (cm -1 M -1) | 250000 1 |
| Excitation (nm) | 656 |
| Emission (nm) | 670 |
| Quantum yield | 0.25 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microscope | |
| Excitation | Cy5 filter set |
| Emission | Cy5 filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 6, 2026

