DiR iodide
1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide
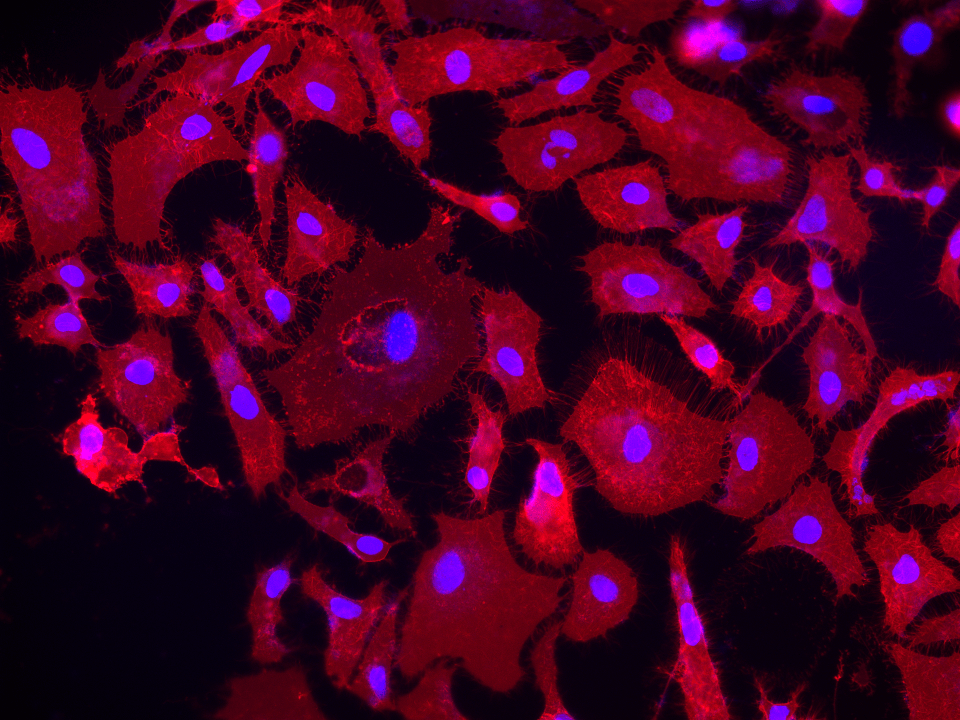
DiR iodide is a lipophilic near-infrared carbocyanine dye that integrates into cell membranes for long-term cell tracking in deep tissue and whole-animal imaging with minimal background fluorescence.
- Near-Infrared Membrane Dye: Ex/Em ~750/780 nm minimizes tissue autofluorescence
- Dual C18 Tail Architecture: Stable membrane integration for long-term cell tracking (weeks)
- Low Cytotoxicity: Minimal phototoxicity and metabolic interference at working concentrations

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22070 | 25 mg | Price |
Overview
DiR dyes are near-infrared (NIR) fluorescent probes engineered for live cell plasma membrane labeling in imaging applications such as confocal microscopy or in vivo small-animal studies. By combining two C18 hydrocarbon tails for stable membrane anchoring with a tricarbocyanine backbone emitting ~775–785 nm, these dyes decrease background autofluorescence and enhances morphological fidelity. Because NIR excitation energy is lower than visible wavelengths, it reduces photobleaching and cellular stress. Carbocyanine analogs with extended hydrocarbon tails can remain in membranes for hours or days, allowing multi-hour time-lapse experiments without re-dyeing. DiR’s emission range also prevents overlap with standard GFP/RFP channels, making multiparametric experiments possible where near-infrared labeling is paired with fluorescent cell cycle reporters. Furthermore, deeper tissue penetration and reduced scattering have been demonstrated in NIR imaging of organs like the colon epithelium, enabling clearer 3D organoid or embryonic system visualizations (Okada, 2013). By bringing together stable bilayer association, lower phototoxicity, and minimal spectral interference with visible fluorophores, DiR stands out as an optimal solution for prolonged, low-background labeling in delicate or complex tissue contexts. Instrument-wise, it is compatible with modern systems that include NIR channels, promoting better tissue penetration and fewer spectral conflicts.
Core Features of DiR
| Structure |
|
| Molecular Weight | Iodide salt: 1 013.39 g/mol—high mass strengthens hydrophobic membrane retention. |
| Color & Spectral Properties |
|
| Lipophilic Dye Mechanism |
|
| Experimental Advantages |
|
Applications
Near-infrared (NIR) labeling of live cells is rapidly expanding into diverse areas of biotechnology research, where deep-tissue imaging and minimal background interference are key. Among NIR lipophilic dyes, DiR stands out for its ability to incorporate into plasma membranes. Below are several cutting-edge fields where DiR is yielding compelling results and driving new breakthroughs in translational medicine.
Stem Cell Therapy and Regenerative Medicine
One of the most active research areas using DiR is tracking stem cell fate and biodistribution. In studies involving rat or mouse models of spinal cord, myocardial, or stroke injury, DiR-labeled mesenchymal stem cells (MSCs) or bone marrow–derived progenitors were injected systemically or locally (Macks et al., 2018; Li et al., 2018). With in vivo NIR imaging, investigators could pinpoint how many labeled cells remained in the target tissue over time. This type of “stem cell homing” analysis is proving instrumental in developing regenerative therapies for previously intractable diseases—such as myocardial infarction or neurodegeneration—while confirming cell viability and retention post-administration.
Immunotherapy and Immune Cell Visualization
In the realm of cancer immunotherapy, DiR labeling enables precise monitoring of immune cells such as T lymphocytes and dendritic cells (Krzastek et al., 2018; Ji et al., 2020). For example, T-cells labeled with DiR can be followed after adoptive transfer to confirm their migration and infiltration into tumors, providing insights into how well they exert their cytotoxic function. Furthermore, dendritic cells labeled with DiR can reveal the dynamics of antigen presentation in lymph nodes and the success of vaccine strategies. This is critical for optimizing protocols in adoptive cell therapy and checkpoint blockade approaches.
Drug Delivery and Nanoparticle Tracking
Another lucrative area centers on nanocarrier systems—for instance, polymeric micelles or liposomes—labeled with DiR for improved visualization (Noack et al., 2020; Zhao et al., 2024). Such NIR imaging clarifies how these carriers distribute throughout the body, whether they accumulate in tumor tissue, and how long they remain in circulation. In parallel, DiR’s hydrophobic chains anchor strongly to phospholipid bilayers, enabling stable association with nanosized vehicles. Researchers leverage this to fine-tune carrier composition and dosing schedules for enhanced therapeutic outcomes. The possibility of real-time, noninvasive imaging of these carriers is helping usher in more effective chemotherapy regimens, targeted tissue repair systems, and advanced gene delivery techniques.
Lymphatic and Vascular Imaging
DiR also finds utility in mapping lymphatic drainage (Wang et al., 2018). This is especially relevant for evaluating metastasis risk or post-surgical complications such as lymphedema. Because DiR-labeled cells or particles can be tracked from peripheral injection sites to lymph nodes, investigators gain crucial spatial and temporal data that influence both cancer staging methods and reconstructive interventions.
Translation to Clinical-Grade Optical Systems
The strong NIR signal from DiR is compatible with many preclinical optical imaging platforms and has the potential to integrate with emerging photoacoustic and hybrid FMT-XCT systems (Berninger et al., 2018). These combined modalities offer deeper tissue penetration, expanded tumor mapping, and precise quantitation of how DiR-labeled cells or nanoparticles distribute within complex 3D tissues.
In summary, DiR’s near-infrared fluorescence, robust membrane integration, and proven safety record make it a transformative tool for cell tracking, immunotherapy optimization, regenerative medicine, nanocarrier evaluation, and beyond. As image-guided technologies advance, DiR continues to foster breakthroughs in biotech and pharmaceutical fields, fueling more reliable research outcomes and guiding the next wave of clinical innovations.
Frequently Asked Questions (FAQ)
1. What is DiR dye?
DiR dye is a near-infrared, lipophilic carbocyanine used primarily to label the plasma membrane of live cells for fluorescence imaging.
2. What is the wavelength of DiR dye?
Typical excitation peaks around 750–760 nm; emission is ~775–785 nm, spanning the near-infrared spectrum.
3. How do lipophilic dyes work?
Lipophilic dyes incorporate into lipid bilayers through hydrophobic alkyl chains, stably tagging cellular membranes without binding to DNA or cytoplasmic proteins.
4. What is the protocol of DiO staining?
DiO is a green-emitting carbocyanine (~484 nm excitation, ~501 nm emission) used similarly:
- Dilute DiO (1–5 µM) in serum-free media.
- Incubate cells (5–20 min, 37 °C).
- Wash away excess dye.
- Proceed with imaging (Honig and Hume, 1989).
5. What is DiO used for?
DiO is used for plasma membrane labeling in multi-color or neuronal tracing studies; its green emission channel pairs well with other fluorophores.
6. What fluorescent dye binds to DNA?
Common nuclear stains (e.g., DAPI, Hoechst 33342, propidium iodide) target DNA, but DiI, DiO, DiD, and DiR remain membrane-selective.
7. What is DiO dye?
DiO is a green lipophilic carbocyanine with octadecyl tails, anchoring into membranes for short- or long-term labeling in fixed or live-cell contexts.
8. How does DiI work?
Similar to DiO, DiI integrates into cell membranes with fluorescence in the orange-red range (~550–565 nm excitation, ~565–595 nm emission) (Chazotte, 2011).
9. What is the best stain for mitochondria?
For mitochondria, dedicated probes such as MitoLite™ or DiOC6(3) are recommended, as general carbocyanines (DiO, DiI, DiR) are less specific (Chen and Coons, 1987).
10. How does DiOC6 stain mitochondria?
DiOC6(3) accumulates in negatively charged inner mitochondrial membranes at low concentrations, selectively highlighting mitochondria (Haugland, 2005).
What is the principle of DiI staining?
DiI relies on the hydrophobic interaction of its alkyl tails with phospholipid bilayers, ensuring stable, uniform membrane labeling (Honig and Hume, 1989).
What is the excitation and emission of DiR dye?
Exact values can vary slightly depending on experimental conditions, but generally:
• Excitation: ~750–760 nm
• Emission: ~775–785 nm
What is the most commonly used fluorescent dye?
Popular visible-spectrum dyes include FITC, TRITC, and Alexa Fluor. For deep-tissue or low-autofluorescence imaging, near-infrared lipophilic dyes (DiI, DiO, DiD, DiR) are indispensable (Chazotte, 2011; Lakowicz, 2006).
Additional Notes
Co-labeling with DNA Synthesis Markers—Although DiR does not monitor proliferation itself, it can be combined with BrdU or EdU to discriminate dividing cells while preserving membrane morphology (Mort et al., 2014).
Deep-Tissue and In Vivo Applications—NIR excitation/emission fosters improved tissue penetration. Studies leveraging near-infrared dyes have shown success in murine models for live tracking of cell migration or tumor homing (Frangioni, 2003).
Multi-channel Imaging—As DiR rarely overlaps with fluorophores that excite <700 nm, you can freely combine it with standard green or red labels to gather multi-parametric data.
Concentration and Incubation—Typical concentration range: 1–10 µM; short exposures (5–20 min) to mitigate nonspecific uptake (Chazotte, 2011).
Safety and Cytotoxicity—Lipophilic dyes like DiR are generally low-toxicity at standard usage levels (Baguley and Lehnert, 1991). Pilot tests are recommended for each cell line.
Comparison of DiI, DiO, DiD and DiR
| Dye | Excitation (nm) | Emission (nm) | Color | Primary Use |
| DiO | ~484 | ~501 | Green | Plasma membrane, neuronal tracing |
| DiI | ~550–565 | ~565–595 | Orange-Red | Long-term cell labeling, tracing |
| DiD | ~646 | ~663 | Far-Red | Deeper tissue imaging |
| DiR | ~750–760 | ~775–785 | Near-Infrared | Low autofluorescence, deep imaging |
All carbocyanine dyes (DiI, DiO, DiD, DiR) employ a similar principle of lipophilic insertion into plasma membranes.
Further Reading
View All
Baguley, Bruce C., and Susan Lehnert. Cell Membrane Labelling with Lipophilic Dyes. Labelling Techniques in Cell Biology, vol. 2, Academic Press, 1991, pp. 75–91.
Barrasso, Anthony P., et al. “Live Imaging of Developing Mouse Retinal Slices.” Neural Development, vol. 13, no. 1, 2018, p. 23, doi:10.1186/s13064-018-0120-y. (Illustrates organotypic live imaging approaches).
Berninger, Markus T., et al. “Detection of Intramyocardially Injected DiR-Labeled Mesenchymal Stem Cells by Optical and Optoacoustic Tomography.” Photoacoustics, vol. 6, 2017, pp. 37–47, doi:10.1016/j.pacs.2017.04.002.
Campanale, Joseph P., et al. “Methods to Label, Isolate, and Image Sea Urchin Small Micromeres, the Primordial Germ Cells (PGCs).” Methods in Cell Biology, vol. 150, 2019, pp. 269–292, doi:10.1016/bs.mcb.2018.11.007. (Discusses various fluorescent labels and live imaging).
Chazotte, Bruce. “Lipophilic Dyes for Membrane Labeling.” Cold Spring Harbor Protocols, 2011, pdb.top66, doi:10.1101/pdb.top66.
Chen, R. F., and A. H. Coons. “Fluorescent Antibody, a New Tool for Immunology.” Experimental Cell Research, vol. 13, no. 2, 1957, pp. 276–278. (Earlier demonstration of fluorescent labeling, conceptual basis).
Frangioni, John V. “In Vivo Near-Infrared Fluorescence Imaging.” Current Opinion in Chemical Biology, vol. 7, no. 5, 2003, pp. 626–634, doi:10.1016/j.cbpa.2003.08.007.
Haugland, Richard P. Handbook of Fluorescent Probes and Research Chemicals. 10th ed., Invitrogen/Molecular Probes, 2005.
Honig, M. G., and R. I. Hume. “DiI and DiO: Versatile Fluorescent Dyes for Neuronal Labeling and Pathway Tracing.” Journal of Neuroscience Methods, vol. 10, no. 1, 1989, pp. 3–14, doi:10.1016/0165-0270(89)90059-8.
Ji, Yuanyuan, et al. “Near-Infrared Fluorescence Imaging in Immunotherapy.” Advanced Drug Delivery Reviews, vol. 158, 2020, pp. 62–82, doi:10.1016/j.addr.2020.06.008.
Kim, Jin Hee, et al. “Zinc Chelation Reduces Hippocampal Neurogenesis after Pilocarpine-induced Seizure.” PLoS ONE, vol. 7, no. 2, 2012, e30691, doi:10.1371/journal.pone.0048543. (Broad use of labeling in a neurological context).
Krzastek, Sarah C., et al. “Dendritic Cell Trafficking in Tumor-Bearing Mice.” Cancer Immunology, Immunotherapy, vol. 67, no. 12, 2018, pp. 1939–1947, doi:10.1007/s00262-018-2187-z.
Lakowicz, Joseph R. Principles of Fluorescence Spectroscopy. 3rd ed., Springer, 2006.
Li, Xiaoli, et al. “Targeted Migration of Bone Marrow Mesenchymal Stem Cells Inhibits Silica-Induced Pulmonary Fibrosis in Rats.” Stem Cell Research & Therapy, vol. 9, no. 1, 2018, p. 335, doi:10.1186/s13287-018-1083-y.
Macks, Christian, et al. “Rolipram-Loaded Polymeric Micelle Nanoparticle Reduces Secondary Injury after Rat Compression Spinal Cord Injury.” Journal of Neurotrauma, vol. 35, no. 3, 2018, pp. 582–592, doi:10.1089/neu.2017.5092.
Mort, Richard L., et al. “Fucci2a: A Bicistronic Cell Cycle Reporter that Allows Cre Mediated Tissue Specific Expression in Mice.” Cell Cycle, vol. 13, no. 17, 2014, pp. 2681–2696, doi:10.4161/15384101.2015.945381. (Demonstrates combination of cell cycle probes with membrane labeling strategies).
Noack, Anne-Kathrin, et al. “Intratumoral Distribution and pH-Dependent Drug Release of High Molecular Weight HPMA Copolymer Drug Conjugates Strongly Depend on Specific Tumor Substructure and Microenvironment.” International Journal of Molecular Sciences, vol. 21, no. 17, 2020, p. 6029, doi:10.3390/ijms21176029.
Wang, Qi-Long, et al. “Blood Exosomes Regulate the Tissue Distribution of Grapefruit-Derived Nanovector via CD36 and IGFR1 Pathways.” Theranostics, vol. 8, no. 18, 2018, pp. 4912–4924, doi:10.7150/thno.27608.
Wu, Dayong, et al. “Determination of DNA Lesion Bypass Using a ChIP-based Assay.” DNA Repair, vol. 108, 2021, p. 103230, doi:10.1016/j.dnarep.2021.103230. (General advanced imaging reference, though not DiR-specific).
Zhao, Xueli, et al. “A Non-Invasive Osteopontin-Targeted Phase Changeable Fluorescent Nanoprobe for Molecular Imaging of Myocardial Fibrosis.” Nanoscale Advances, vol. 6, no. 14, 2024, pp. 3590–3601, doi:10.1039/d4na00042k.
Calculators
Common stock solution preparation
Table 1. Volume of DMSO needed to reconstitute specific mass of DiR iodide [1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide] to given concentration. Note that volume is only for preparing stock solution. Refer to sample experimental protocol for appropriate experimental/physiological buffers.0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
1 mM | 98.679 µL | 493.393 µL | 986.787 µL | 4.934 mL | 9.868 mL |
5 mM | 19.736 µL | 98.679 µL | 197.357 µL | 986.787 µL | 1.974 mL |
10 mM | 9.868 µL | 49.339 µL | 98.679 µL | 493.393 µL | 986.787 µL |
Molarity calculator
Enter any two values (mass, volume, concentration) to calculate the third.Citations
View all 160 citations: Citation Explorer
ICAM-1 promotes T cell glycolytic reprogramming and tumor infiltration to drive 18F-FDG PET flares following radiotherapy
Authors:
Song, Rui and Zhao, Meixin and Zhang, Ting and Zhang, Yining and Guo, Fuxin and He, Huiying and Zhou, Haoyi and Li, Kui and Wang, Jianze and Du, Jinhong and others,
Journal:
Protein \& Cell (2025): pwaf111
PACS-2 Mitigates NPSC Apoptosis and Intervertebral Disc Degeneration by Preserving MAM Integrity via the SP1/LRRK2/Mfn2 Axis
Authors:
Kang, Liang and Wang, Jiaqi and Zhao, Chenhao and Li, Qiuwei and Zhang, Zhigang and Zhang, Huaqing and Jia, Chongyu and Zhou, Luping and Wang, Yanxin and Chen, Yu and others,
Journal:
Advanced Science (2025): e11781
A fibrin gel-loaded Gouqi-derived nanovesicle (GqDNV) repairs the heart after myocardial infarction by inhibiting p38 MAPK/NF-$\kappa$B p65 pathway
Authors:
Zhou, Huan-Huan and Zhou, Xiaolei and Pei, Jianqiu and Xu, Shiyin and Jin, Biyu and Chen, Jiuling and Zhang, Zixuan and Tang, Mingmeng and Liu, Yan and N{\"u}ssler, Andreas K and others,
Journal:
Journal of Nanobiotechnology (2025): 1--23
Quercetin-Loaded Mesenchymal Stem Cell Derived Extracellular Vesicles Enhance Ovarian Function in a Cyclophosphamide Induced Ovarian Damage
Authors:
Korun, Zeynep Ece Utkan and Halbutogullari, Zehra Seda and Yazir, Yusufhan and Furat, Selenay and Altuntas, Candan and Ozturk, Ahmet and Koldankaya, Tugba and Mert, Serap and Attar, Erkut
Cancer-associated fibroblast-derived extracellular vesicles loaded with GLUT1 inhibitor synergize anti-PD-L1 to suppress tumor growth via degrading matrix stiffness and remodeling tumor microenvironment
Authors:
Wu, Yali and Chen, Wenjuan and Deng, Jingjing and Zhou, Xingyu and Chen, Jiangbin and Yang, Zimo and Cao, Xinghui and Liu, Jiatong and Tan, Qi and Zhou, E and others,
Journal:
Journal of Controlled Release (2025): 113998
References
View all 92 references: Citation Explorer
Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing
Authors:
Kalchenko V, Shivtiel S, Malina V, Lapid K, Haramati S, Lapidot T, Brill A, Harmelin A.
Journal:
J Biomed Opt (2006): 50507
Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius
Authors:
Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C.
Journal:
Hypertension (2006): 482
Functional neuroanatomy of the rhinophore of Aplysia punctata
Authors:
Wertz A, Rossler W, Obermayer M, Bickmeyer U.
Journal:
Front Zool (2006): 6
In vivo imaging and counting of rat retinal ganglion cells using a scanning laser ophthalmoscope
Authors:
Higashide T, Kawaguchi I, Ohkubo S, Takeda H, Sugiyama K.
Journal:
Invest Ophthalmol Vis Sci (2006): 2943
Confocal laser scanning microscopy using dialkylcarbocyanine dyes for cell tracing in hard and soft biomaterials
Authors:
Heinrich L, Freyria AM, Melin M, Tourneur Y, Maksoud R, Bernengo JC, Hartmann DJ.
Journal:
J Biomed Mater Res B Appl Biomater. (2006)
Physical properties
| Molecular weight | 1013.39 |
| Solvent | DMSO |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 270000 1 |
| Excitation (nm) | 754 |
| Emission (nm) | 778 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
| CAS | 100068-60-8 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 16, 2026

