Cell Meter™ Fluorescence Gap Junction Tracing Kit
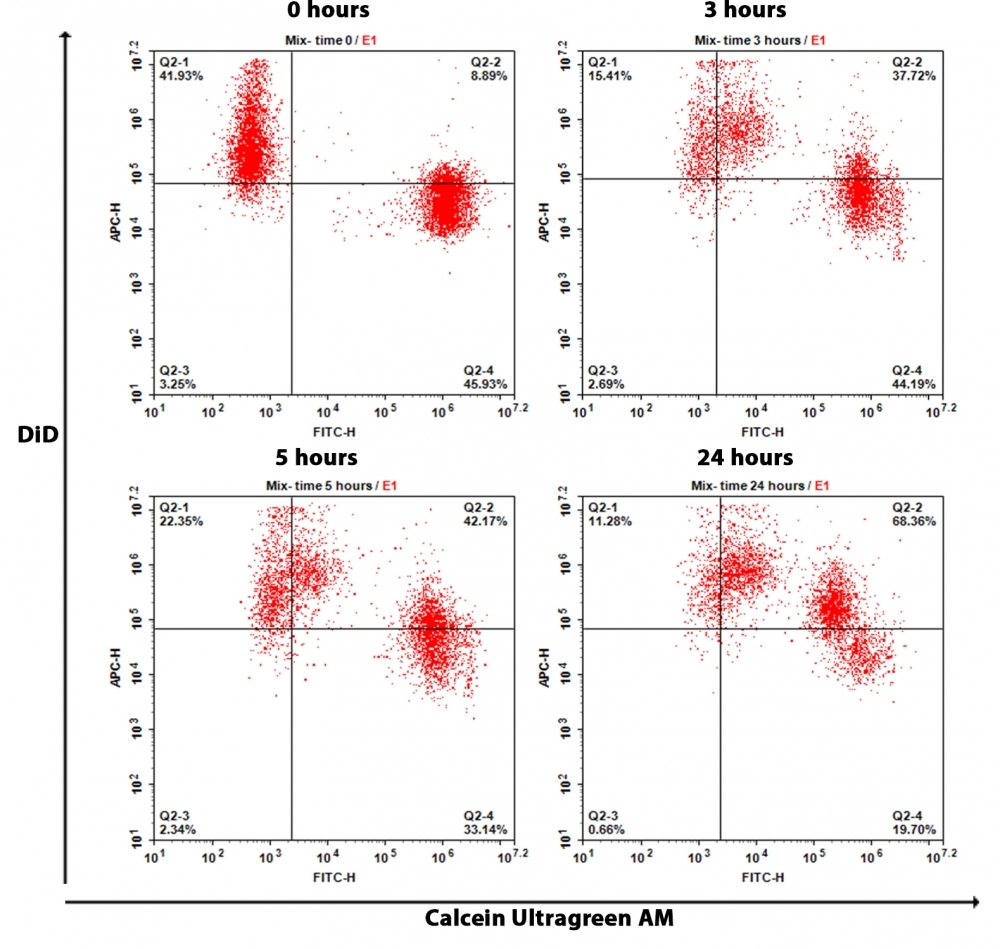
Gap junctions are specialized intercellular membrane channels constituted with connexin that selectively facilitate the passage of small molecules of <1.5 kD across cells. They are tightly regulated by voltage, growth factors, cAMP, and retinoids, and they are modulated by phosphorylation. Cell Meter™ Fluorescence Gap Junction Tracing Kit provides a reliable and robust assay for the in vitro determination of gap junction function. The method is noninvasive. The cell population under study is divided such that one fraction is loaded with a lipophilic cell plasma membrane permeable dye, Calcein UltraGreen™ AM, that is hydrolyzed upon cellular uptake by cytoplasmic esterases to yield Calcein UltraGreen, a highly fluorescent and well-retained and membrane-impermeable molecule. The other fraction is loaded DiD, which is a lipophilic membrane dye that diffuses laterally to stain the entire cell membrane in deep red fluorescence upon incorporation into membranes. The two fractions are mixed and incubated under coculture conditions. Calcein UltraGreen is transferred to the DiD-stained cells through gap junctions. The assessment of this uptake can be monitored by fluorescence imaging or flow cytometry.
Example protocol
AT A GLANCE
Protocol summary
- Add Calcein Ultragreen AM working solution to the cells
- Add DiD working solution to the cells
- Incubate at 37 °C for 10-30 minutes
- Wash cells with GAP Junction Assay Buffer
- Resuspend cells in cell culture medium and mix them with 1:1 ratio
- Measure the fluorescence signal at various times using a flow cytometer with 530/30 nm and 660/20 nm emission filters or a fluorescence microscope with FITC and Cy5 filter sets
Important
Thaw all the kit components at room temperature before starting the experiment.PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
Note Store the unused Calcein Ultragreen AM stock solution at -20 °C in single use aliquots to avoid freeze thaw cycles.
Note One vial is enough for 50 tests.
Note Store the unused DiD stock solution at -20 °C in single use aliquots to avoid freeze thaw cycles.
1. Calcein Ultragreen AM stock solution
Add 50 µL of DMSO (Component D) into one Calcein Ultragreen AM vial (Component A) and mix well.Note Store the unused Calcein Ultragreen AM stock solution at -20 °C in single use aliquots to avoid freeze thaw cycles.
Note One vial is enough for 50 tests.
2. DiD stock solution
Add 100 µL of DMSO (Component D) into DiD (Component B) and mix well.Note Store the unused DiD stock solution at -20 °C in single use aliquots to avoid freeze thaw cycles.
PREPARATION OF WORKING SOLUTION
1. Calcein Ultragreen AM working solution
Add 1 µL of Calcein Ultragreen AM stock solution to 1 mL of GAP Junction Assay Buffer and mix well.Note Calcein Ultragreen AM working solution should not be stored and should be used promptly.
Note 1 mL Calcein Ultragreen AM working solution is enough for two tests.
2. DiD working solution
Add 1 µL of DiD stock solution into 1 mL of GAP Junction Assay Buffer and mix well.Note DiD working solution should not be stored and should be used promptly.
Note 1 mL DiD working solution is enough for two tests.
SAMPLE EXPERIMENTAL PROTOCOL
The following protocol can be used as a guideline and should be optimized according to the needs.
Cell staining protocol for Calcein Ultragreen AM
- Grow cells in cell culture medium in 6-well cell culture plates.
- Remove the cell culture medium and add 0.5 mL of Calcein Ultragreen AM working solution.
- Incubate cells at 37 °C for 10-20 minutes.
Note Incubation time should be optimized for each cell line. - Remove the dye working solution and wash cells with GAP Junction Assay buffer.
Note For the adherent cells, detach cells from the plate using rubber policeman. - Resuspend cells in cell culture medium.
Cell labelling protocol for DiD
- Grow cells in cell culture medium in 6-well cell culture plates.
- Remove the cell culture medium and add 0.5 mL of DiD working solution.
- Incubate cells at 37 °C for 10-20 minutes.
Note Incubation time should be optimized for each cell line. - Remove the dye working solution and wash cells with GAP Junction Assay Buffer.
Note For the adherent cells, detach cells from the plate using rubber policeman. - Resuspend cells in cell culture medium.
GAP junction assay
- Mix Calcein stained cells and DiD labelled cells with 1:1 ratio and plate them in wells.
Note For the fluorescence microscopy, add 50 µL of each into the well of a 96-well plate and mix well.
Note For the flow cytometer, add 500 µL of each into the well of a 6-well plate and mix well. - Incubate cells at 37 °C for 2-3 hours.
- Monitor the cells with a fluorescence microscope using the FITC and Cy5 filter sets.
- For flow cytometric analysis: For adherent cells, detach cells using rubber policeman. Once cells are in suspension or for cells in suspension, wash cells twice with DPBS or buffer of your choice. Resuspend cells in HHBS (cat# 20011), or DPBS or buffer of your choice and measure response with 530/30 nm filter (FITC channel) and 660/20 nm filter (APC channel).
Citations
View all 1 citations: Citation Explorer
Macrophages enhance sodium channel expression in cardiomyocytes
Authors: Bogert, NV and Therre, M and Din, S and Furkel, J and Zhou, X and El-Battrawy, I and Heineke, J and Schweizer, PA and Akin, I and Katus, HA and others,
Journal: Basic Research in Cardiology (2024): 1--11
Authors: Bogert, NV and Therre, M and Din, S and Furkel, J and Zhou, X and El-Battrawy, I and Heineke, J and Schweizer, PA and Akin, I and Katus, HA and others,
Journal: Basic Research in Cardiology (2024): 1--11
References
View all 50 references: Citation Explorer
Regulation of connexin 43 by interleukin 1β in adult rat cardiac fibroblasts and effects in an adult rat cardiac myocyte: fibroblast co-culture model.
Authors: McArthur, Lisa and Riddell, Alexandra and Chilton, Lisa and Smith, Godfrey L and Nicklin, Stuart A
Journal: Heliyon (2020): e03031
Authors: McArthur, Lisa and Riddell, Alexandra and Chilton, Lisa and Smith, Godfrey L and Nicklin, Stuart A
Journal: Heliyon (2020): e03031
Cilostamide and forskolin maintain gap junction function of incubated dog follicles.
Authors: Thongkittidilok, Chommanart and Doriguzzi, Nicole and Nagashima, Jennifer and Brown, Megan and Chansaenroj, Ajjima and Songsasen, Nucharin
Journal: Theriogenology (2020): 222-228
Authors: Thongkittidilok, Chommanart and Doriguzzi, Nicole and Nagashima, Jennifer and Brown, Megan and Chansaenroj, Ajjima and Songsasen, Nucharin
Journal: Theriogenology (2020): 222-228
Granulosa secreted factors improve the developmental competence of cumulus oocyte complexes from small antral follicles in sheep.
Authors: Rouhollahi Varnosfaderani, Shiva and Hajian, Mehdi and Jafarpour, Farnoosh and Ghazvini Zadegan, Faezeh and Nasr-Esfahani, Mohammad Hossein
Journal: PloS one (2020): e0229043
Authors: Rouhollahi Varnosfaderani, Shiva and Hajian, Mehdi and Jafarpour, Farnoosh and Ghazvini Zadegan, Faezeh and Nasr-Esfahani, Mohammad Hossein
Journal: PloS one (2020): e0229043
G protein-coupled receptor 30 mediates meiosis resumption and gap junction communications downregulation in goat cumulus-oocyte complexes by 17β-estradiol.
Authors: Zhang, Hui and Wei, Qiang and Gao, Zhen and Ma, Chiyuan and Yang, Zhenshan and Zhao, Hui and Liu, Chen and Liu, Jie and Zhao, Xiaoe and Ma, Baohua
Journal: The Journal of steroid biochemistry and molecular biology (2019): 58-67
Authors: Zhang, Hui and Wei, Qiang and Gao, Zhen and Ma, Chiyuan and Yang, Zhenshan and Zhao, Hui and Liu, Chen and Liu, Jie and Zhao, Xiaoe and Ma, Baohua
Journal: The Journal of steroid biochemistry and molecular biology (2019): 58-67
An Assay to Assess Gap Junction Communication in Cell Lines.
Authors: Warawdekar, Ujjwala M
Journal: Journal of biomolecular techniques : JBT (2019): 1-6
Authors: Warawdekar, Ujjwala M
Journal: Journal of biomolecular techniques : JBT (2019): 1-6
Page updated on March 11, 2025