Calcein Deep Red™ acetate
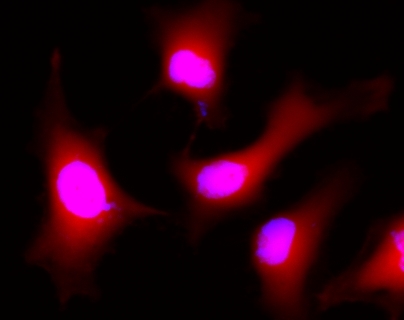
This product is now replaced by #22011. Calcein AM is one the most popular fluorescent probes used for labeling and monitoring cellular functions of live cells. However, the single color of Calcein AM makes it impossible to use this valuable reagent in the multicolor applications. For example, it is impossible to use Calcein AM in combination of GFP-tranfacted cells due to the same color to GFP. To address this color limitation of Calcein AM, we have developed Calcein Orange™, Calcein Red™ and Calcein Deep Red™. These new Calcein AM analogs enable the multicolor labeling and functional analysis of live cells in combination with Calcein AM. Non-fluorescent Calcein Deep Red™ acetate can easily get into live cells and hydrolyzes to generate strongly fluorescent Calcein Deep Red™ (Cat#: 21902) dye. Calcein Deep Red™ dye can be monitored with the common Cy5 filter set.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22010 | 1 mg | Price |
Physical properties
| Molecular weight | 530.36 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 643 |
| Emission (nm) | 663 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 640 nm laser |
| Emission | 660/20 nm filter |
| Instrument specification(s) | APC channel |
| Fluorescence microscope | |
| Excitation | Cy5 filter set |
| Emission | Cy5 filter set |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 620 |
| Emission | 660 |
| Cutoff | 630 |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 23, 2025

