Amplite® Fluorimetric Glutathione GSH/GSSG Ratio Assay Kit *Green Fluorescence*
When cells are exposed to increased levels of oxidative stress, GSSG will accumulate and the ratio of GSH to GSSG will decrease. The glutathione redutase recycles GSSG to GSH with simultaneous oxidation of b-nicotinamide adenine dinuclecotide phosphate. The monitoring of GSH/GSSG ratio and the quantification of GSSG in biological samples are essential for evaluating the redox and detoxification status of cells and tissues in relation to the protective role of glutathione against oxidative and free-radical-mediated cell injury. There are a few reagents or assay kits available for the quantitation of thiols in biological systems. However, all the commercial kits either lack sensitivity or have tedious protocols. Our Amplite® Fluorimetric GSH/GSSG Ratio Kit provides an ultrasensitive assay to quantitate GSH in the sample. The kit uses a proprietary non-fluorescent dye that becomes strongly fluorescent upon reacting with thiol. The kit provides a sensitive, one-step fluorimetric method to detect as little as 1 picomole of cysteine or GSH in a 100 µL assay volume. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation without a separation step. Its signal can be easily read by a fluorescence microplate reader.
Example protocol
AT A GLANCE
Important With regards to GSH:GSSG determination, the following equation is important to remember:
GSSG -> Reduction -> 2 GSH
For each redox of GSSG, two moles of GSH is produced. Thus, when determining [Total GSH] during data analysis, it is important to remember that [Total GSH] = 2 x [GSSG]. For instance, if [GSSG] standard for a particular well is 5 uM, [Total GSH] for that well should be calculated using 10 uM.
GSSG -> Reduction -> 2 GSH
For each redox of GSSG, two moles of GSH is produced. Thus, when determining [Total GSH] during data analysis, it is important to remember that [Total GSH] = 2 x [GSSG]. For instance, if [GSSG] standard for a particular well is 5 uM, [Total GSH] for that well should be calculated using 10 uM.
Protocol Summary
- Prepare GSH standards and/or GSSG standards or test samples (50 µL)
- Add GSH working solution (50 µL)
- Incubate at RT for 10 to 60 minutes
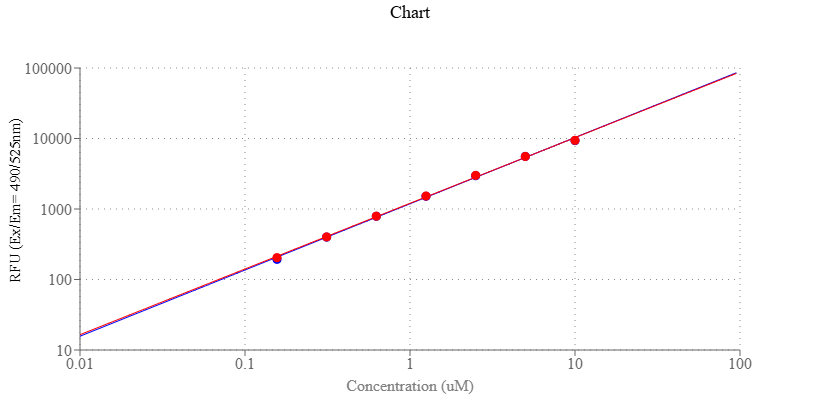
- Monitor the fluorescence increase at Ex/Em = 490/520 nm
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
1. GSH standard solution (1 mM)
Add 200 µL of Assay Buffer (Component B) into the vial of GSH Standard (Component C) to make 1 mM (1 nmol/µL) GSH standard solution.2. GSSG standard solution (1 mM)
Add 200 µL of ddH2O into the vial of GSSG Standard (Component F) to make 1 mM (1 nmol/µL) GSSG standard solution.3. Thiolite™ Green stock solution (100X)
Add 100 µL of DMSO (Component D) into the vial of Thiolite™ Green (Component A) to make 100X Thiolite™ Green stock solution. Note: One might add 200 µL of DMSO to make 50 X Thiolite™ Green stock solution for better solubility.PREPARATION OF STANDARD SOLUTION
For convenience, use the Serial Dilution Planner:
https://www.aatbio.com/tools/serial-dilution/10056
https://www.aatbio.com/tools/serial-dilution/10056
GSH or GSSG standard
Prepare serially diluted GSH standards (0 to 10 μM): Add 10μL of GSH standard stock solution to 990 μL of Assay Buffer (Component B) to generate 10 μM GSH standard solution (GSH7). Note: Diluted GSH standard solution is unstable. Use within 4 hours. Take 10 μM GSH standard solution to perform 1:2 serial dilutions to get 5, 2.5, 1.25, 0.625, 0.3125, and 0.1563 serially diluted GSH standards (GSH6-GSH1). Prepare serially diluted GSSG standards (0 to 5 μM):Add 10μL of GSSG standard stock solution into 990 μL of Assay Buffer (Component B) to generate 10 μM GSSG standard solution. Note: Diluted GSSG standard solution is unstable. Use within 4 hours. Take 10 μM GSSG standard solution to perform 1:2 serial dilutions to get 5, 2.5, 1.25, 0.625, 0.3125, 0.1563, and 0.0781 serially diluted GSSG standards (GSSG7-GSSG1).PREPARATION OF WORKING SOLUTION
1. GSH working solution (GSH-WS)
Add 100 μL of 100X Thiolite™ Green stock solution into 10 mL of Assay Buffer (Component B) and mix well by vortexing. Note: This GSH working solution (GSH-WS) is enough for two 96-well plates. It is unstable at room temperature, and should be used promptly within 2 hours. Note: Avoid exposure to light.[/note]Note Alternatively, one can make GSH working solution by adding 100X Thiolite™ Green stock solution with Assay Buffer proportionally.
2. Total GSH working solution (TGSH-WS)
Add 5 mL of GSH-WS into the bottle of GSSG Probe (Component E) and mix them well by vortexing. Note: This Total GSH working solution (TGSH-WS) is enough for one 96-well plates. It is unstable at room temperature, and should be used promptly within 2 hours and avoid exposure to light. Note: Alternatively, one can make a 25X GSSG Probe by adding 200 μL of ddH2O into the bottle of Component E, and then prepare the TGSH-WS by mix the stock solution with GSH-WS proportionally.SAMPLE EXPERIMENTAL PROTOCOL
Table 1. Layout of GSH standards, GSSG standards and test samples in a solid black 96-well microplate. GSH = GSH Standards (GSH1 - GSH7, 0.15 to 10 µM); GSSG = GSSG Standards (GSSG1 - GSSG7, 0.08 to 5 µM); BL=Blank Control; TS=Test Samples
Table 2. Reagent composition for each well.
| Panel A | Pannel B | ||||||
| BL | BL | TS | TS | BL | BL | TS | TS |
| GSH1 | GSH1 | ... | ... | GSSG1 | GSSG1 | ... | ... |
| GSH2 | GSH2 | ... | ... | GSSG2 | GSSG2 | ... | ... |
| GSH3 | GSH3 | GSSG3 | GSSG3 | ||||
| GSH4 | GSH4 | GSSG4 | GSSG4 | ||||
| GSH5 | GSH5 | GSSG5 | GSSG5 | ||||
| GSH6 | GSH6 | GSSG6 | GSSG6 | ||||
| GSH7 | GSH7 | GSSG7 | GSSG7 | ||||
| Well | Volume | Reagent |
| GSH | 50 µL | Serial Dilutions (0.15 to 10 µM) |
| GSSG | 50 µL | Serial Dilutions (0.08 to 5 µM) |
| BL | 50 µL | Assay Buffer |
| TS | 50 µL | Test Sample |
- Prepare GSH standards (GSH), blank controls (BL), and test samples (TS) according to the layout provided in Tables 1 and 2. For a 384-well plate, use 25 µL of reagent per well instead of 50 µL.
- Add 50 µL of GSH-WS into each well of GSH standards, blank controls and test samples to make the total assay volume 100 µL/well. Use 25 µL for 384-well plates, for total assay volume of 50 µL/well.
- If Total GSH (in reduced and oxidized states) assay is needed, prepare TGSH-WS and GSSG standards. Add GSSG standards (GSSG), blank controls (BL), and test samples (TS) according to layout provided in Tables 1 and 2. For a 384-well plate, use 25 µL of reagent per well instead of 50 µL. Then add 50 µL of TSGH-WS into each well of GSSG standards, blank controls and test samples to make the total assay volume 100 µL/well (50 µL total assay volume for 384-well plates).
- Incubate the reaction for 10 to 60 minutes at room temperature, protected from light.
- Monitor the fluorescence increase at Ex/Em = 490/520 nm with a fluorescence microplate reader.
Citations
View all 16 citations: Citation Explorer
A cotton APS REDUCTASE represses root hair elongation via sulfur assimilation and hydrogen peroxide-mediated cell wall damage
Authors: Mao, Xiaonan and Wei, Zhenzhen and Xu, Zhenzhen and Qanmber, Ghulam and Liu, Ji and Li, Yonghui and Lu, Lili and Wang, Ye and Peng, Jun and Wang, Zhi
Journal: Physiologia Plantarum (2024): e14200
Authors: Mao, Xiaonan and Wei, Zhenzhen and Xu, Zhenzhen and Qanmber, Ghulam and Liu, Ji and Li, Yonghui and Lu, Lili and Wang, Ye and Peng, Jun and Wang, Zhi
Journal: Physiologia Plantarum (2024): e14200
YAP promotes the healing of ischemic wounds by reducing ferroptosis in skin fibroblasts through inhibition of ferritinophagy
Authors: Cao, Guoqi and Yin, Siyuan and Ma, Jiaxu and Lu, Yongpan and Song, Ru and Wu, Zhenjie and Liu, Chunyan and Liu, Jian and Wu, Peng and Sun, Rui and others,
Journal: Heliyon (2024)
Authors: Cao, Guoqi and Yin, Siyuan and Ma, Jiaxu and Lu, Yongpan and Song, Ru and Wu, Zhenjie and Liu, Chunyan and Liu, Jian and Wu, Peng and Sun, Rui and others,
Journal: Heliyon (2024)
The physiological and biochemical effects on Napier grass plants following Napier grass stunt phytoplasma infection
Authors: Asudi, George Ochieng and Omenge, Keziah Moraa and Paulmann, Maria K and Reichelt, Michael and Grabe, Veit and Mithofer, Axel and Oelmueller, Ralf and Furch, Alexandra CU
Journal: Phytopathology (2020)
Authors: Asudi, George Ochieng and Omenge, Keziah Moraa and Paulmann, Maria K and Reichelt, Michael and Grabe, Veit and Mithofer, Axel and Oelmueller, Ralf and Furch, Alexandra CU
Journal: Phytopathology (2020)
Analysis of the Status of the Cutaneous Endogenous and Exogenous Antioxidative System of Smokers and the Short-Term Effect of Defined Smoking Thereon
Authors: Lohan, Silke B and B{\"u}hring, Karl and Lauer, Anna-Christina and Friedrich, Annette and Lademann, J{\"u}rgen and Buss, Annette and Sabat, Robert and Wolk, Kerstin and Meinke, Martina C
Journal: Antioxidants (2020): 537
Authors: Lohan, Silke B and B{\"u}hring, Karl and Lauer, Anna-Christina and Friedrich, Annette and Lademann, J{\"u}rgen and Buss, Annette and Sabat, Robert and Wolk, Kerstin and Meinke, Martina C
Journal: Antioxidants (2020): 537
Glutathione S-transferase theta 1 protects against colitis through goblet cell differentiation via interleukin-22
Authors: Kim, Jae Hyeon and Ahn, Jae Bum and Kim, Da Hye and Kim, Soochan and Ma, Hyun Woo and Che, Xiumei and Seo, Dong Hyuk and Kim, Tae Il and Kim, Won Ho and Cheon, Jae Hee and others,
Journal: The FASEB Journal (2020)
Authors: Kim, Jae Hyeon and Ahn, Jae Bum and Kim, Da Hye and Kim, Soochan and Ma, Hyun Woo and Che, Xiumei and Seo, Dong Hyuk and Kim, Tae Il and Kim, Won Ho and Cheon, Jae Hee and others,
Journal: The FASEB Journal (2020)
Page updated on April 15, 2025