iFluor® 488-Wheat Germ Agglutinin (WGA) Conjugate
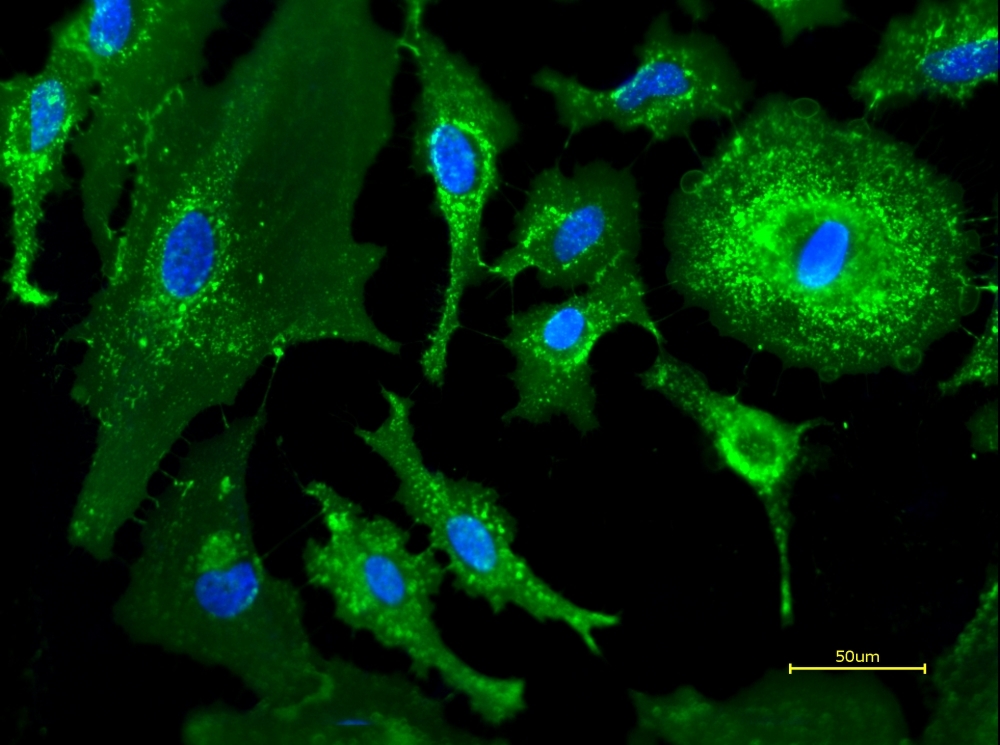
iFluor® 488-WGA conjugate is a green-fluorescent wheat germ agglutinin that selectively binds N-acetylglucosamine and sialic acid residues, enabling specific labeling of cell membranes, fibrotic tissue, and fungal cell walls.
- Glycoconjugate Specificity: Binds N-acetylglucosamine and N-acetylneuraminic acid residues
- Bright Green Fluorescence: iFluor® 488 provides superior photostability vs FITC (Ex/Em = 491/514 nm)
- Membrane Labeling: 36 kDa lectin labels plasma membrane and fibrotic tissue
- Fungal Cell Wall Marker: Specific for chitin-containing structures in yeast and fungi

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 25530 | 1 mg | Price |
Physical properties
| Solvent | Water |
Spectral properties
| Correction factor (260 nm) | 0.21 |
| Correction factor (280 nm) | 0.11 |
| Extinction coefficient (cm -1 M -1) | 75000 1 |
| Excitation (nm) | 491 |
| Emission (nm) | 516 |
| Quantum yield | 0.9 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 20, 2026

