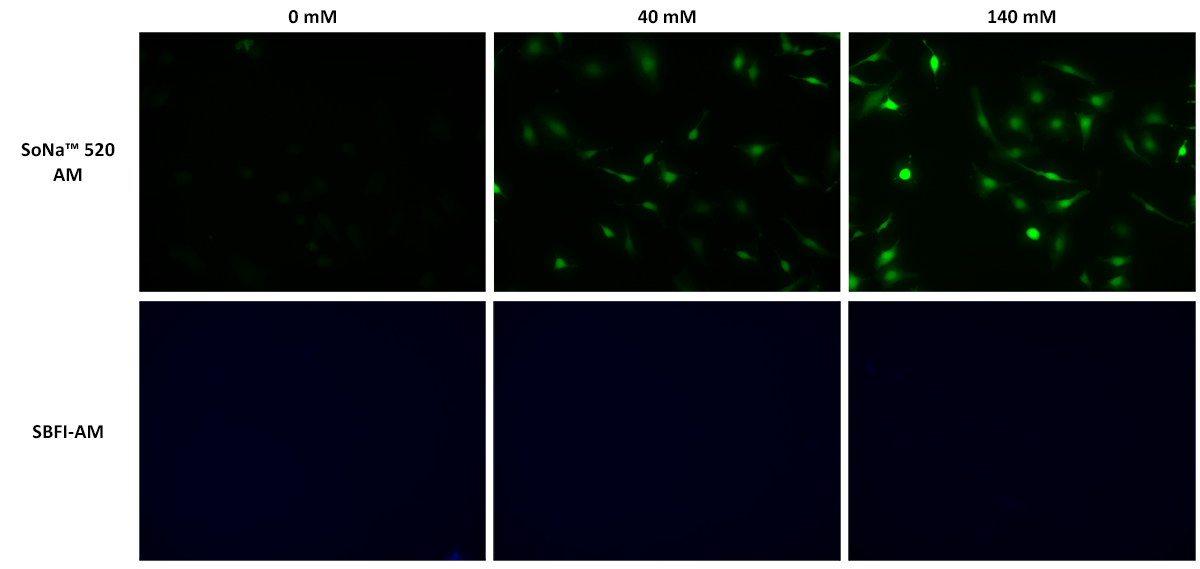
SoNa™ 520 AM is the cell-permeable version of SoNa™ 520 used for monitoring sodium ions in live cells. SoNa™ 520 is a new sodium-sensitive fluorescent indicator dye used to detect sodium levels in biological samples. It is a fluorescent dye that undergoes a great enhancement in fluorescence intensity upon binding to sodium ions. By measuring the emitted fluorescence intensities, researchers can assess the sodium ion concentration within a biological sample or study how sodium ion changes upon a biological stimulation. It perhaps has the highest detection sensitivity compared to other well-known fluorescent sodium ion indicators such as SBFI and Corona Red. It can be employed to measure changes in sodium concentration in living cells and other biological samples. Compared to the most common SBFI, SoNa™ 520 is much more sensitive, with a much larger fluorescence response under the same conditions. In addition, SoNa™ 520 can be well excited with the visible 488 nm laser or similar visible light to avoid the UV excitation that is required for exciting SBFI. In general, UV excitation causes great damage to cells and other biological samples and also photobleaches the dye probes much more quickly than visible light. The use of a sodium ion indicator allows scientists to investigate various physiological processes related to sodium, such as sodium ion channel activity, cell signaling, and sodium homeostasis. SoNa™ 520 provides valuable insights into cellular mechanisms and can be utilized in fields like neuroscience, cardiology, and cellular biology.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 21323 | 1 mg | Price | |
| 21324 | 10x50 ug | Price |
| Molecular weight | 804.8 |
| Solvent | DMSO |
| Excitation (nm) | 491 |
| Emission (nm) | 511 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Fluorescence microscope | |
| Excitation | FITC |
| Emission | FITC |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 490 |
| Emission | 525 |
| Cutoff | 515 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |

