Screen Quest™ TR-FRET No Wash cAMP Assay Kit
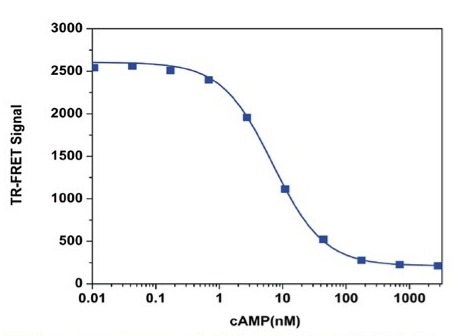
Screen Quest™ TR-FRET No Wash cAMP Assay Kit provides a convenient assay method for monitoring the activation of adenylyl cyclase in G-protein coupled receptor systems. Compared to other commercial ELISA cAMP assay kits, this homogenous cAMP assay kit does not require a wash step or the acetylation step. The assay is based on the competition for a fixed number of anti-cAMP antibody binding sites between the trFluor™ 650 labeled cAMP tracer and non-labeled free cAMP. The anti-cAMP antibody is labeled with trFluor™ Eu while the cAMP tracer is labeled with trFluor™ 650. In the absence of cAMP, trFluor™ 650-cAMP conjugate is bound to trFluor™ Eu-labeled anti-cAMP antibody exclusively to have a strong FRET signal. While the unlabeled free cAMP is present in the test sample, it competes for the trFluor™ Eu-labeled anti-cAMP antibody conjugate binding sites, therefore inhibits the binding of trFluor™ 650-cAMP to anti-cAMP antibody. The trFluor™ 650 labeled cAMP tracer only has fluorescence lifetime of nanosecond while TR Fluor™ Eu-labeled anti-cAMP antibody-bound fluorescent cAMP tracer has much longer fluorescence lifetime of lanthanide fluorophore. The magnitude of time -resolved fluorescence signal (TR- FRET) signal is proportional to the concentration of cAMP in a sample. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format, and is convenient for monitoring the cAMP activity with ultra-specificity and sensitivity in G-protein coupled receptor systems.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 36379 | 1 plate | Price | |
| 36380 | 10 plates | Price | |
| 36381 | 50 plates | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Recommended plate | Solid black and/or Black wall/clear bottom |
| Instrument specification(s) | Time-resolved |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 13, 2026
