Nociceptin/Orphanin FQ Peptide Receptor (NOP) is a member of the opioid receptor family that is involved in regulating pain, anxiety, stress, and other physiological processes. Unlike classical opioid receptors, NOP signaling does not mediate typical opioid-induced analgesia, euphoria and respiratory depression, making it an attractive target for developing safer therapeutics for pain management, addiction, and neurological disorders. Traditional methods for studying GPCRs often fail to capture the complex signaling mechanisms of specific receptors like NOP, which modulate intracellular cAMP levels.
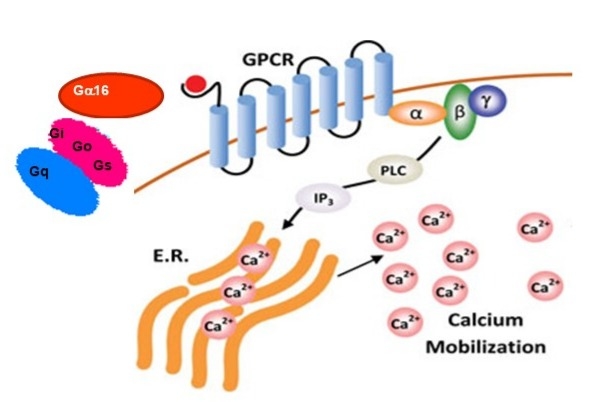
The Screen Quest™ Live Cell Opioid-Like Receptor NOP cAMP Assay Service Pack is specifically designed for real-time, high-throughput monitoring of intracellular cAMP changes associated with NOP receptor activation. This assay employs transfected cell lines co-expressing NOP and a promiscuous G-protein Gα16. The co-expression of Gα16 enables NOP to couple to Gq signaling, resulting in calcium mobilization. Activation of NOP by specific ligands, such as nociceptin/orphanin FQ, can be detected using calcium-sensitive dyes like Calbryte™ 520 AM, Cal-520® AM, Fluo-8® AM, Fluo-4™ AM, or other compatible no-wash calcium kits.
This service pack provides all necessary components for precise measurement of NOP receptor-mediated cAMP changes using FLIPR, FDSS, or equivalent fluorescence microplate readers. It is an excellent tool for researchers studying NOP signaling pathways and evaluating potential therapeutic compounds targeting this receptor, particularly in the context of pain, addiction, and stress-related disorders.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 38207 | 100 Tests | Price |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Black wall/Clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
