Screen Quest™ Live Cell Chemokine (CC) receptor CCR5 cAMP Assay Service Pack
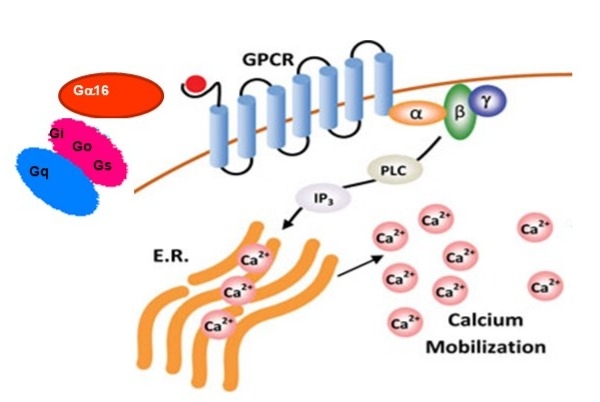
Screen Quest™ Live Cell CCR5 cAMP Assay Service Pack monitors CCR5 receptor signaling important for HIV infection and inflammatory diseases through live-cell cAMP detection.
- Real-Time cAMP Monitoring: Enables precise, live-cell measurement of intracellular cAMP changes in response to CCR5 activation without requiring cell lysis
- Convenient: Provides all necessary components for precise measurement of CCR5-mediated cAMP changes
- High-Throughput: Optimized for use with FLIPR, FDSS, and other fluorescence microplate readers, making it ideal for large-scale screening of CCR5-targeting compounds
- Simple Workflow: Utilizes wash-free calcium sensitive dyes reducing assay complexity and time

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 38203 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Black wall/Clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 19, 2026
