ReadiUse™ TCEP removal solution
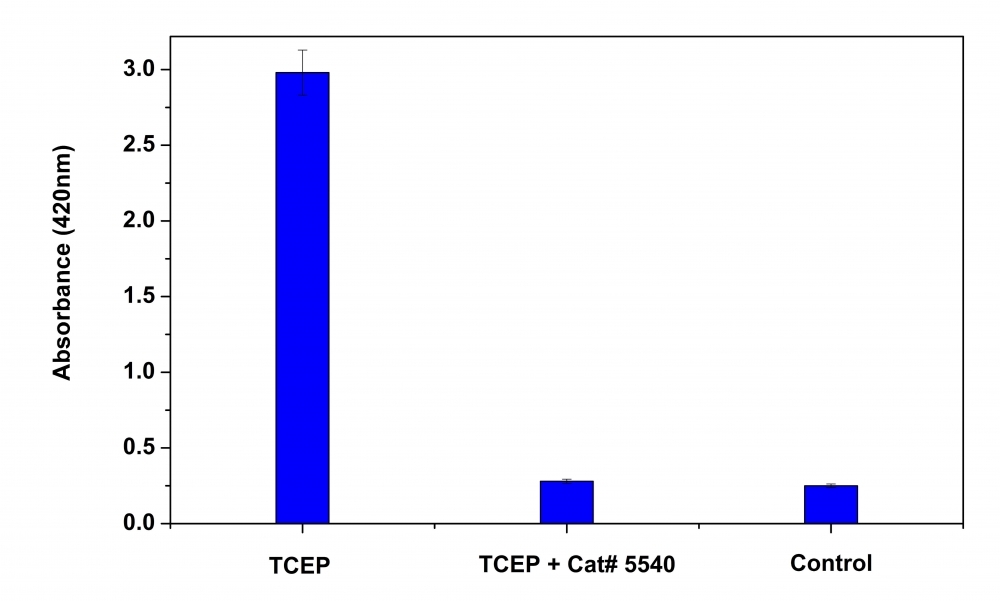
TCEP is a common reducing reagent widely used to break disulfide bonds within and between proteins. Compared to the other two most common reducing agents, i.e., dithiothreitol (DTT) and 2-mercaptoethanol, TCEP has the advantages of being odorless, a more powerful reducing agent, an irreversible reducing agent, more hydrophilic, and more resistant to oxidation in air. TCEP is also used in the tissue homogenization process for RNA isolation. However, there are numerous reports that TCEP is not compatible with downstream assays that often have components reactive to TCEP. Our ReadiUse™ TCEP removing buffer can be effectively used for eliminating residual TCEP. It is a preformatted 2M solution that can be simply added to a solution that contains residual TCEP.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5540 | 1 ml | Price |
Physical properties
| Molecular weight | N/A |
| Solvent | Water |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 13, 2026
