ReadiUse™ TCA Deproteinization Sample Preparation Kit
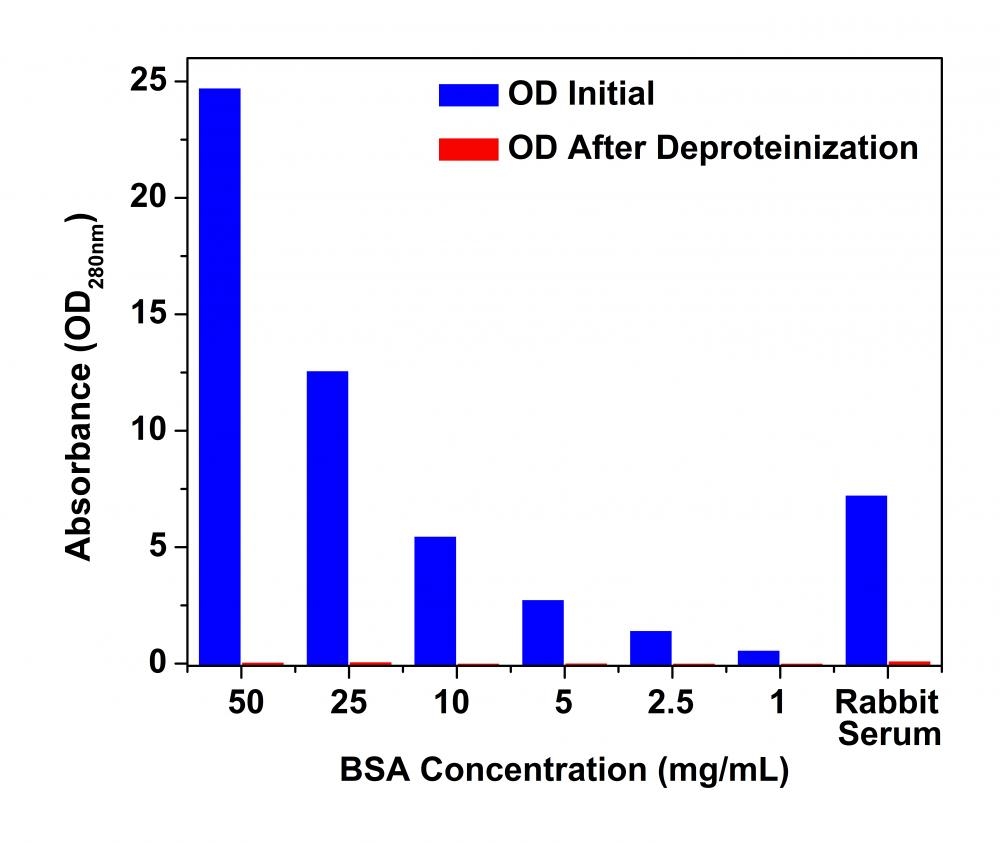
The analysis of amino acids, ions, metabolites and other small molecules is frequently hindered by the interference of lipids, protein and enzymes present in biofluids, cell and tissue lysates. Therefore, deproteinization is a necessary step in many procedures prior to analysis of biological samples. AAT Bioquest's Deproteinization Sample Preparation Kit offers a rapid method for removing proteins in biological samples. Proteins are precipitated by applying trichloroacetic acid (TCA). After removal of precipitated proteins, the pH of the sample is neutralized with neutralization solution. TCA based deproteinization has been successfully and widely used in sample preparation prior to quantitation of small molecules, such as glycogen, ATP, cAMP, glutathione and antioxidants. The TCA method provided in this kit can also be used for removing protein in large scale. Biological samples prepared using this kit can be directly used in various biochemical analysis.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 19501 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H340 |
| Hazard symbol | T |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R68 |
| UNSPSC | 41116134 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 20, 2026
