ReadiUse™ Preactivated PerCP-Cy5.5 Tandem
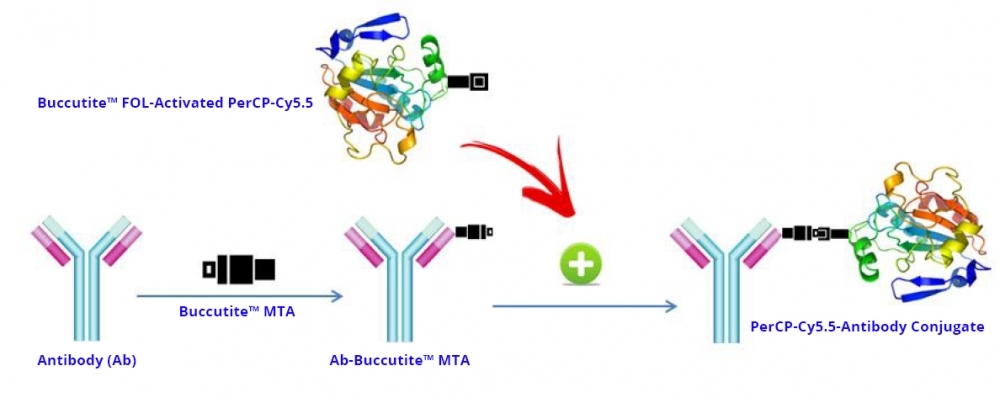
PerCP (Peridinin-chlorophyll-protein complex) is isolated from Dinophyceae sp. It has an extremely high extinction coefficient, a high quantum efficiency and a large Stokes shift. It is well excited with the Argon laser at 488 nm with its maximum emission peak at 677 nm. PerCP protein is commonly used for fluorescent immunolabeling, particularly in applications involving fluorescent-activated cell sorting (FACS). Its tandem conjugates (such as PerCP-Cy5.5) can be excited with a standard 488 nm laser and emits in the far red at a longer wavelength for multicolor flow cytometric analysis of cells. These multiple emission wavelengths make PerCP- Cyanine conjugates potentially useful fluorochromes for multicolor analysis with FITC, PE and other fluorochromes. PerCP tandem structure may make it more photostable than PerCP alone, which generally photobleaches rapidly with more powerful water-cooled gas lasers. AAT Bioquest offers this preactivated PerCP-Cy5.5 Tandem to facilitate the PerCP-Cy5.5 tandem conjugations to antibodies and other proteins such as streptavidin and other secondary reagents. Our preactivated PerCP-Cy5.5 tandem is ready to conjugate, giving much higher yield than the conventionally tedious SMCC-based conjugation chemistry. In addition, our preactivated PerCP-Cy5.5 tandem is conjugated to a protein via its amino group that is abundant in proteins while SMCC chemistry targets the thiol group that has to be regenerated by the reduction of antibodies.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 2595 | 1 mg | Price |
Physical properties
| Solvent | Water |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 350000 |
| Excitation (nm) | 482 |
| Emission (nm) | 695 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Refrigerated (2-8 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 25, 2026

