ReadiLink™ Rapid mFluor™ Violet 610 Antibody Labeling Kit
Microscale Optimized for Labeling 50 µg Antibody Per Reaction
ReadiLink™ Rapid mFluor™ Violet 610 Antibody Labeling Kits provide a quick and convenient method to label antibodies, proteins (>10 kDa), or other amine-containing biomolecules. Leveraging a unique conjugation chemistry, ReadiLink™ Antibody Labeling Kits enables researchers to label microscale volumes of antibodies in two easy steps, with no purification needed and 100% recovery of the labeled product. Each kit includes sufficient mFluor™ Violet 610 dye to facilitate two distinct labeling reactions of 50 µg antibody samples. The resulting conjugates are ideal for a wide range of applications, including flow cytometry, fluorescent microscopy techniques, immunocytochemistry (ICC), immunohistochemistry (IHC), ELISA, and indirect FISH. Compared to Alexa Fluor dyes and conventional dye counterparts, mFluor™ dyes are brighter, more photostable, and resistance to changes in pH between pH 4 to 10. The mFluor™ Violet 610 dyes are optimally excited by violet lasers and emit red fluorescence ~610 nm. These properties make them well-suited for spectral fluorescence flow cytometry, providing researchers with a tool for detailed biological analysis with unparalleled precision and sensitivity. With ReadiLink™ Rapid Antibody Labeling kits, researchers can directly label primary antibodies, eliminating the need for secondary antibodies and enhancing panel-building flexibility.
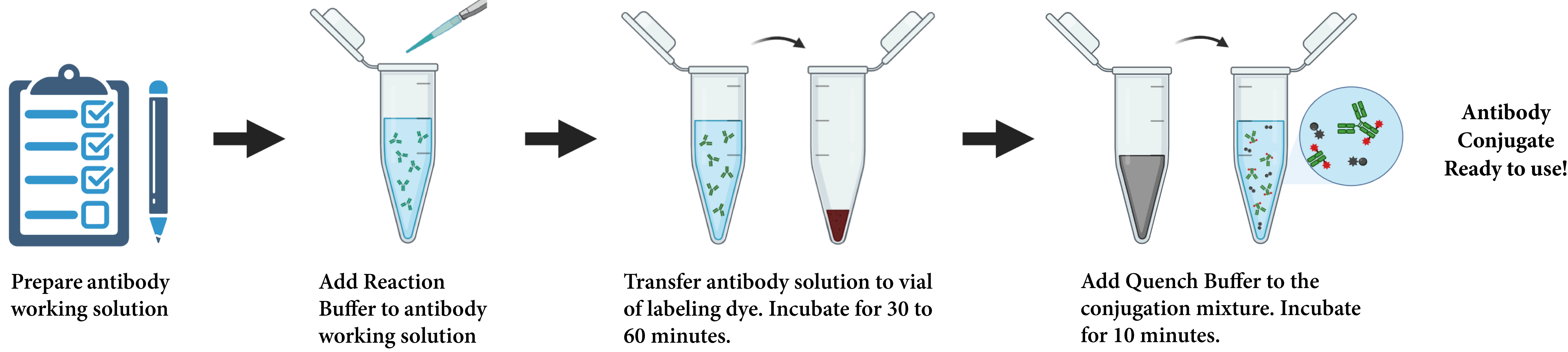
Figure 1. Overview of the ReadiLink™ Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 1116 | 2 Labelings | Price |
Spectral properties
| Absorbance (nm) | 594 |
| Correction factor (260 nm) | 0.532 |
| Correction factor (280 nm) | 0.66 |
| Extinction coefficient (cm -1 M -1) | 90000 1 |
| Excitation (nm) | 421 |
| Emission (nm) | 612 |
| Quantum yield | 0.3 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 12, 2026

