ReadiLink™ Rapid iFluor® 750 Antibody Labeling Kit *Microscale Optimized for Labeling 50 μg Antibody Per Reaction*
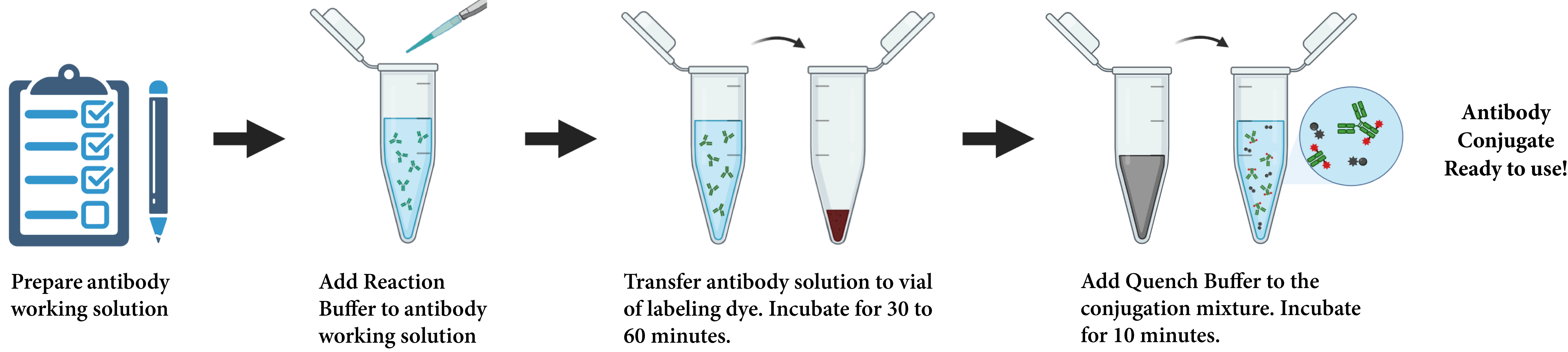
Figure 1. Overview of the ReadiLink™ Rapid Antibody Labeling protocol. In just two simple steps, and with no purification necessary, covalently label microgram amounts of antibodies in under an hour.
Example protocol
AT A GLANCE
Warm all the components and centrifuge the vials briefly before opening them. Immediately prepare the necessary solutions before starting your conjugation. The following protocol is a recommendation.
PREPARATION OF WORKING SOLUTION
For labeling 50 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 5 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 50 µL of the target protein solution.
Note: If you have a different protein concentration, adjust the protein volume accordingly to make ~50 µg of protein available for your labeling reaction.
Note: For labeling 100 µg of protein (assuming the target protein concentration is 1 mg/mL), mix 10 µL (10% of the total reaction volume) of Reaction Buffer (Component B) with 100 µL of the target protein solution.
Note: The protein should be dissolved in 1X phosphate buffered saline (PBS), pH 7.2 - 7.4; if the protein is dissolved in glycine buffer, it must be dialyzed against 1X PBS, pH 7.2 - 7.4, or use Amicon Ultra-0.5, Ultracel-10 Membrane, 10 kDa (cat# UFC501008 from Millipore) to remove free amines or ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation.
Note: Impure antibodies or antibodies stabilized with bovine serum albumin (BSA) or gelatin will not be labeled well.
Note: A final protein concentration range of 1 - 2 mg/mL is recommended for optimal labeling efficiency, with a significantly reduced conjugation efficiency at less than 1 mg/mL.
SAMPLE EXPERIMENTAL PROTOCOL
Add the protein working solution (Solution A) to ONE vial of labeling dye (Component A), and mix them well by repeatedly pipetting for a few times or vortex the vial for a few seconds.
Note: If labeling 100 µg of protein, use both vials (Component A) of labeling dye by dividing the 100 µg of protein into 2 x 50 µg of protein and reacting each 50 µg of protein with one vial of labeling dye. Then combine both vials for the next step.
Keep the conjugation reaction mixture at room temperature for 30 - 60 minutes.
Note: The conjugation reaction mixture can be rotated or shaken for a longer time if desired.
- Add 5 µL (for 50 µg protein) or 10 µL (for 100 µg protein) which is 10% of the total reaction volume of TQ™-Dyed Quench Buffer (Component C) into the conjugation reaction mixture; mix well.
- Incubate at room temperature for 10 minutes. The labeled protein (antibody) is now ready to use.
The protein conjugate should be stored at > 0.5 mg/mL in the presence of a carrier protein (e.g., 0.1% bovine serum albumin). For longer storage, the protein conjugates could be lyophilized or divided into single-used aliquots and stored at ≤ –20°C.
Spectrum
Product family
Citations
Authors: Zhou, Yuhan and Ye, Ting and Ye, Chengzhi and Wan, Chao and Yuan, Siyue and Liu, Yushuai and Li, Tianyu and Jiang, Fagang and Lovell, Jonathan F and Jin, Honglin and others,
Journal: Bioactive Materials (2021)
Authors: Gao, D and Liu, N and Li, Y and Zhang, Y and Liu, G and others, undefined
Journal: Metabolomics (Los Angel) (2017): 2153--0769
Authors: Wu, Kenneth and Chong, Robert A and Yu, Qing and Bai, Jin and Spratt, Donald E and Ching, Kevin and Lee, Chan and Miao, Haibin and Tappin, Inger and Hurwitz, Jerard and others, undefined
Journal: Proceedings of the National Academy of Sciences (2016): E2011--E2018
Authors: Piccard, Helene and Berghmans, Nele and Korpos, Eva and Dillen, Chris and Aelst, Ilse Van and Li, S and ra , undefined and Martens, Erik and Liekens, S and ra , undefined and Noppen, Sam and Damme, Jo Van and others, undefined
Journal: International Journal of Cancer (2012): E425--E436
References
Authors: Sadhu KK, Mizukami S, Watanabe S, Kikuchi K.
Journal: Mol Biosyst (2011): 1766
Authors: Zhang S, Tan HC, Ooi EE.
Journal: J Vis Exp. (2011)
Authors: Arai S, Yoon SI, Murata A, Takabayashi M, Wu X, Lu Y, Takeoka S, Ozaki M.
Journal: Biochem Biophys Res Commun (2011): 211
Authors: Cui JJ, Zhu XL, Ji CF, Jing XH, Bai WZ.
Journal: Zhen Ci Yan Jiu (2011): 262
Authors: Kuwayama M, Shigemoto N, Oohara S, Tanizawa Y, Yamada H, Takeda Y, Matsuo T, Fukuda S.
Journal: J Microbiol Methods (2011): 119



