Power Styramide™ Signal Amplification (PSA) system is one of the most sensitive fluorescence imaging methods that can detect extremely low-abundance targets in cells and tissues with improved fluorescence signal 10-50 times higher than the commonly used tyramide (TSA) reagents. ReadiCleave™ Styramides are the new iteration of our PSA™ products that add the reversible capability to chemically remove the PSA™ staining on tissue or in cells if needed. As our other PSA™ reagents, the ReadiCleave™ Styramides are an excellent collection of multicolor reagents to simultaneously detect multiple targets in the same tissue samples. They provide an additional benefit, i.e., the PSA™ staining can be removed (if desired) while preserving the integrity of tissue samples. A specific protein is first recognized by its selective primary antibody. Subsequently, the target is stained by the HRP-secondary antibody conjugate of its immunoglobulin class. The bright red, fluorescent ReadiCleave ™ iFluor® 546 Styramide is added subsequently. The HRP-antibody conjugate catalyzes the coupling reaction between ReadiCleave™ iFluor® 546 Styramide and the target protein in proximity. The red iFluor® 546 Styramide staining can be gently removed with nearly 100% efficiency using ReadiCleave™ AML Cleavage Buffer when needed.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 45510 | 100 Slides | Price |
| Molecular weight | 1666.09 |
| Solvent | DMSO |
| Correction factor (260 nm) | 0.25 |
| Correction factor (280 nm) | 0.15 |
| Extinction coefficient (cm -1 M -1) | 100000 1 |
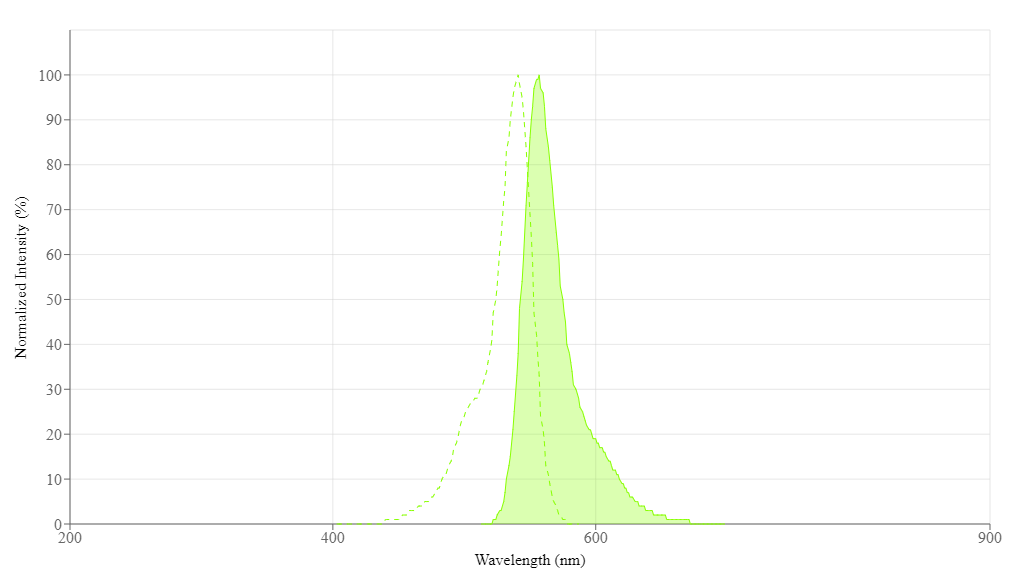
| Excitation (nm) | 541 |
| Emission (nm) | 557 |
| Quantum yield | 0.67 1 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| Fluorescence microscope | |
| Excitation | FITC filter set |
| Emission | FITC filter set |
| Recommended plate | Black wall/clear bottom |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |

