mFluor™ Blue 660 tyramide is a fluorescent labeling reagent used in immunofluorescence staining and in situ hybridization. Tyramide is a small molecule that can diffuse through tissue sections or cell membranes and subsequently be enzymatically amplified to produce a localized, highly fluorescent signal. The mFluor™ Blue 660 dye is conjugated to tyramide to create a fluorescently labeled tyramide that can be used for visualizing specific target molecules or structures within biological samples. mFluor™ Blue 660 tyramide has the largest Stokes Shift among all the commercial tyramide reagents. The high sensitivity and specificity of mFluor™ Blue 660 tyramide make it an excellent choice for fluorescence imaging and detection in various histochemical fluorescence imaging applications.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11051 | 200 Slides | Price |
| Molecular weight | 770.00 |
| Solvent | DMSO |
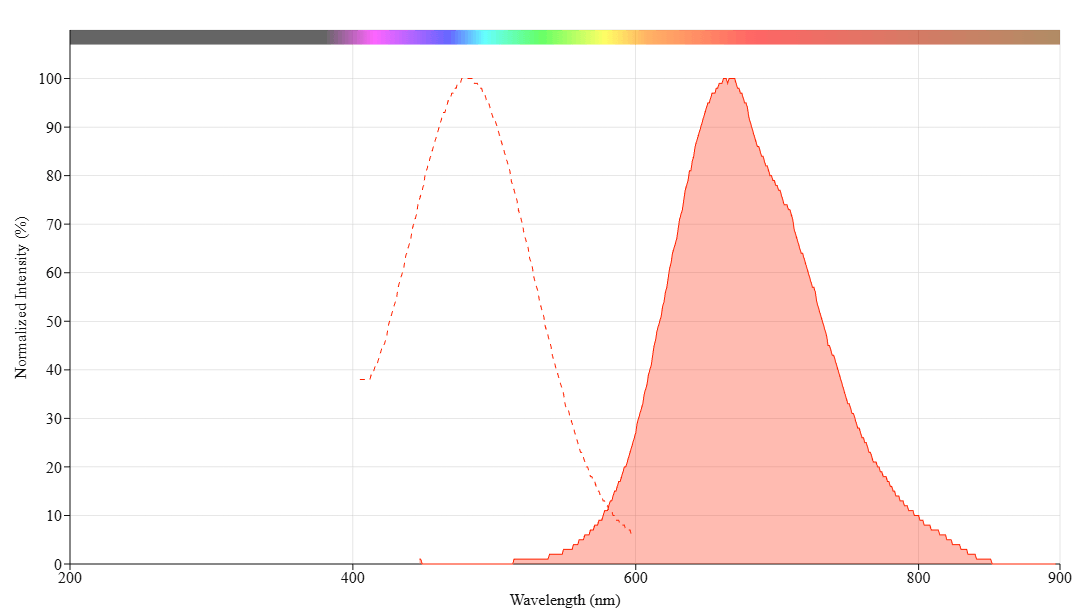
| Absorbance (nm) | 481 |
| Correction factor (260 nm) | 0.338 |
| Correction factor (280 nm) | 0.32 |
| Extinction coefficient (cm -1 M -1) | 26000 1 |
| Excitation (nm) | 481 |
| Emission (nm) | 663 |
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
| Fluorescence microscope | |
| Excitation | Violet filter set |
| Emission | Violet filter set |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Compatible with Cy5 filter set |
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |

