Live or Dead™ Cell Viability Assay Kit
Green/Red Dual Fluorescence
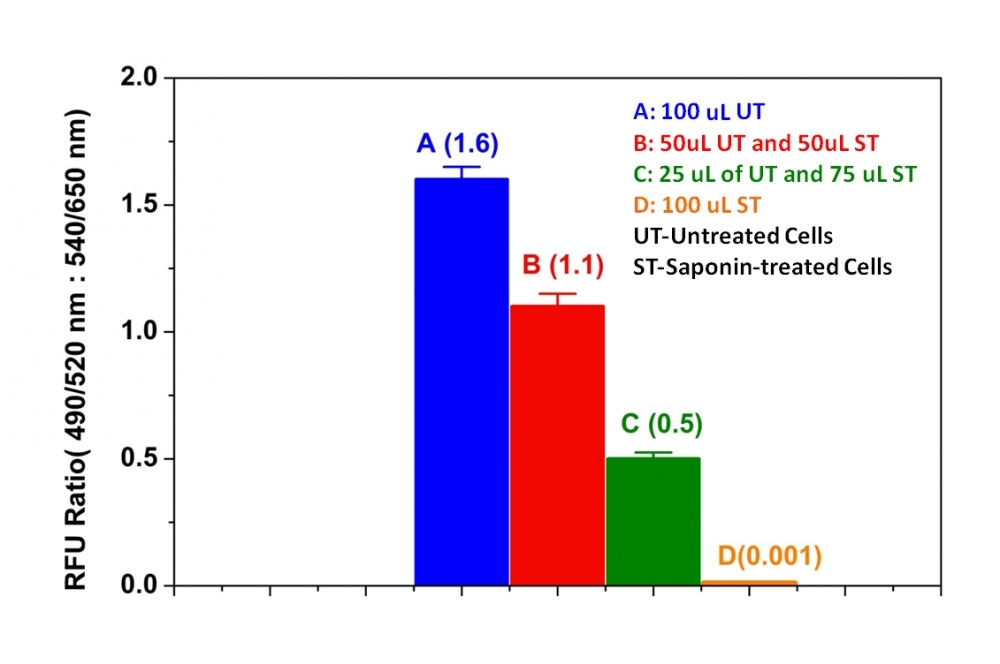
This Live or Dead™cell viability uses two fluorogenic indicators: calcein AM for viable cells and a cell-impermeable DNA-binding dye for the cells with compromised membranes. Calcein AM is a hydrophobic compound that easily permeates intact live cells, and becomes strongly fluorescent upon hydrolysis by esterases. The hydrolysis of the non-fluorescent calcein AM by intracellular esterases generates the strongly fluorescent hydrophilic calcein that is well-retained in the cell cytoplasm. The esterase activity is proportional to the number of vial cells. The DNA-binding dye is quite polar and impermeable for viable cells that have intact membranes. It becomes fluorescent only upon binding to DNA of dead cells. Cells grown in black-walled plates can be stained and quantified in less than two hours. The assay is more robust and accurate than the other viability assays. It can be readily adapted for high-throughput assays in a wide variety of fluorescence platforms such as microplate assays, immunocytochemistry and flow cytometry. The kit provides all the essential components with an optimized assay protocol. It is suitable for proliferating and non-proliferating cells, and can be used for both suspension and adherent cells. Using 100 ul of reagents per well in a 96-well format, this kit provides sufficient reagents to perform 100 assays. Using 25 ul of reagents per well in a 384-well format, this kit provides sufficient reagents to perform 400 assays.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22760 | 1000 Tests | Price | |
| 22789 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm, 610/20 nm filter |
| Instrument specification(s) | FITC, PE-Texas Red channel |
| Fluorescence microscope | |
| Excitation | FITC filter (live), TRITC filter (dead) |
| Emission | FITC filter (live), TRITC filter (dead) |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 490 nm (live), 540 nm (dead) |
| Emission | 525 nm (live), 620 nm (dead) |
| Cutoff | 515 nm, 590 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on August 5, 2024
