FluoroQuest™ PLUS Antifade Mounting Medium
Example protocol
AT A GLANCE
Prepare Samples (slides or microplate wells).
Add a drop of a component and mount.
Examine the specimen under microscope.
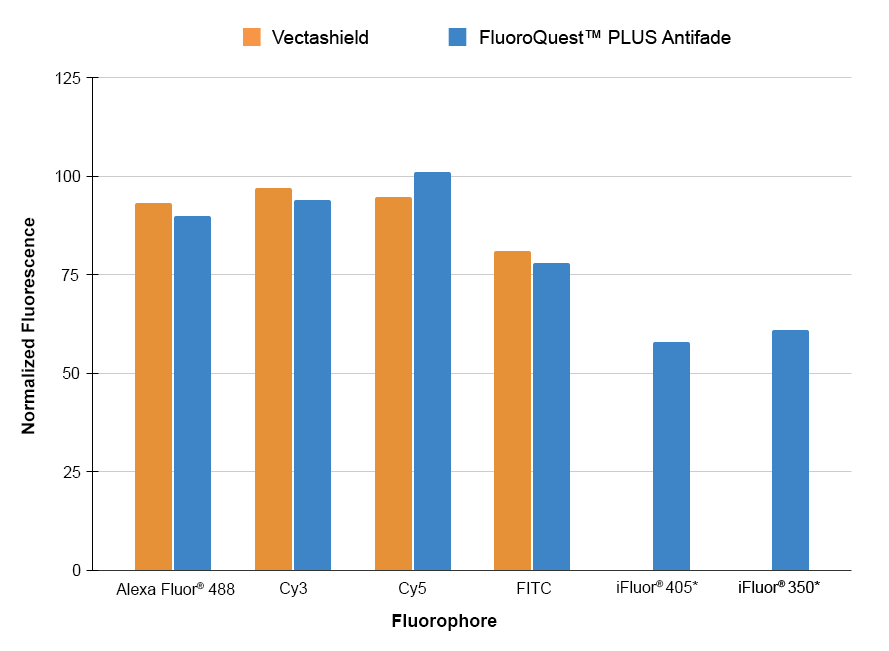
These ready-to-use anti-fading reagents can be applied directly to the washed specimen. Although the reagents have been tested with lots of fixed samples, their optimal anti-fading efficiencies strongly depend on the properties of your samples. We suggest that you try more than one component for your imaging samples to get the ideal component. For example, one component may be more compatible with a fluorescently labeled antibody conjugate (or an enzyme substrate or special mounting specimens that contain lipophilic plasma membrane stains like DiI) than another one.
CELL PREPARATION
For guidelines on cell sample preparation, please visit:
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
SAMPLE EXPERIMENTAL PROTOCOL
Thaw the vial of FluoroQuest™ PLUS Antifade Mounting Medium at room temperature, protected from light.
Remove any excess liquid from your specimen. Add a small drop of the selected component to the specimen. If the sample is on a slide or tissue culture dish, carefully place a coverslip on the drop, avoiding air bubbles. If the sample is on a coverslip, invert the coverslip on a clean glass slide. Remove any excess anti-fading component.
The anti-fading reagents should be incubated for 1 hour to overnight. For long-term storage, seal the coverslip to the slide with nail polish or a plastic sealant. Mounted slides should be stored at 4°C in the dark for optimum sample longevity. The fluorescence imaging would remain stable for many weeks. Samples can be imaged immediately after mounting.