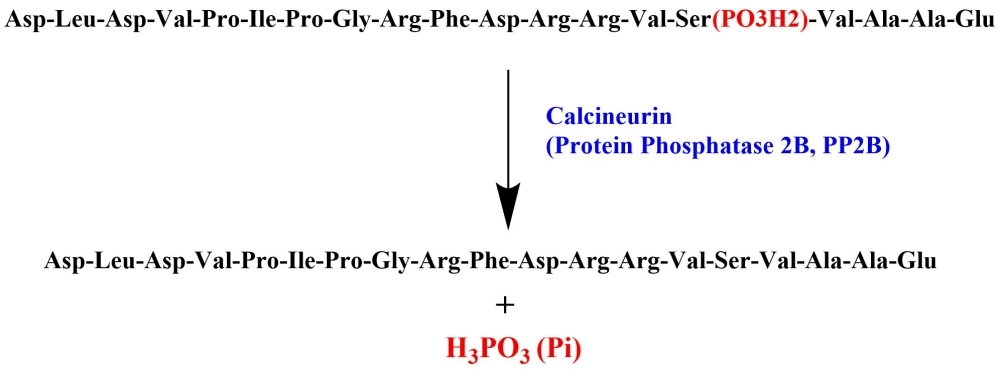
DLDVPIPGRFDRRVpSVAAE
DLDVPIPGRFDRRVpSVAAE (H-Asp-Leu-Asp-Val-Pro-Ile-Pro-Gly-Arg-Phe-Asp-Arg-Arg-Val-Ser(PO₃H₂)-Val-Ala-Ala-Glu-OH) is an excellent peptide substrate for calcineurin (Protein Phosphatase 2B, PP2B). The sequence is derived from the PKA regulatory subunit type II (RII) and is phosphorylated on the Ser residue. Calcineurin is a calcium-dependent, serine/threonine phosphatase that is involved in a variety of signaling pathways. Calcineurin is distinct among phosphatases because its activity requires calcium and is not sensitive to inhibition by compounds that block the related phosphatases PP1A and PP2A. The current common techniques quantify calcineurin activity by measuring released radioactive phosphate or detection of free phosphate with malachite green. Both methods involve technical challenges and have undesirable features. AAT Bioquest has developed a few excellent phosphate assays that can be used for monitoring the release of phosphate from the dephosphorylation of DLDVPIPGRFDRRVpSVAAE by calcineurin. PhosphoWorks™ Colorimetric Phosphate Assay Kit (21665) and PhosphoWorks™ Colorimetric MESG Phosphate Assay Kit (21659) can be used for the colorimetric measurement of phosphate. For higher sensitivity, the release of phosphate can be quantified fluorometrically with PhosphoWorks™ Fluorimetric Phosphate Assay Kit (21660).

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 31700 | 1 mg | Price |
Physical properties
| Molecular weight | 2192.34 |
| Solvent | DMSO |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 24, 2026
