Cyanine 3 monosuccinimidyl ester, potassium salt
same as GE Cy3® NHS ester
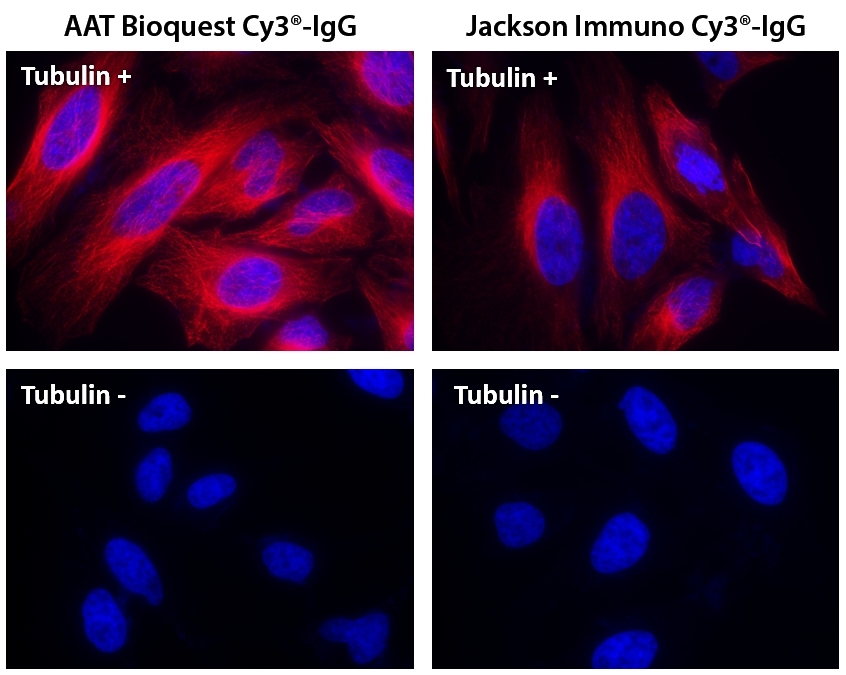
This Cy3® dye is the same molecule to GE's Cy3® NHS ester. Cy3® NHS ester readily reacts with amino groups. A variety of cyanine 3 (Cy3®) dyes has been used to label biological molecules for fluorescence imaging and other fluorescence-based biochemical analysis. They are widely used for labeling peptides, proteins and oligos etc. Cy3® dyes have enhanced fluorescence upon binding to proteins. Cy3® is the trademark of GE Healthcare.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 271 | 1 mg | Price |
Physical properties
| Molecular weight | 765.93 |
| Solvent | DMSO |
Spectral properties
| Correction factor (260 nm) | 0.07 |
| Correction factor (280 nm) | 0.073 |
| Extinction coefficient (cm -1 M -1) | 150000 1 |
| Excitation (nm) | 555 |
| Emission (nm) | 569 |
| Quantum yield | 0.15 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12171501 |
| CAS | 146368-16-3 |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 14, 2026

