Cell Meter™ Phosphatidylserine Apoptosis Assay Kit
Green Fluorescence Excited at 405 nm
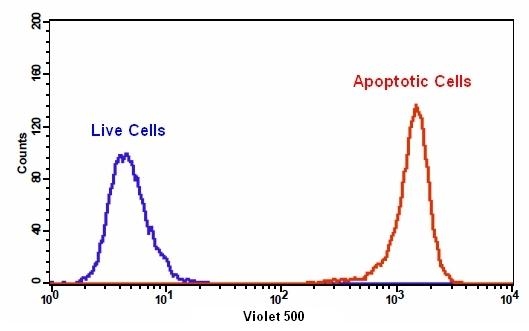
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used for monitoring cell viability. This particular kit is designed to monitor cell apoptosis through measuring the translocation of phosphatidylserine (PS). In apoptosis, PS is transferred to the outer leaflet of the plasma membrane. The appearance of phosphatidylserine on the cell surface is a universal indicator of the initial/intermediate stages of cell apoptosis and can be detected before morphological changes can be observed. Our proprietary Apopxin™ PS sensor used in this kit is small molecule-based PS sensor. The green fluorescent stain is well excited with the Violet Laser at 405 nm, and emits intense green fluorescence at ~520 nm. The kit is optimized to be used with a flow cytometer equipped with Violet Laser. It is particularly suitable for multicolor flow cytometric analysis of cells. In coupling with its large Stokes Shift, its highly enhanced affinity to PS makes this kit more robust than the other commercial Annexin V based apoptosis kits that are only used with either microscope or flow cytometry platform. This kit can be also used with a fluorescence microplate reader besides the microscope and flow cytometry platforms.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22836 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 405 nm laser |
| Emission | 525/40 nm filter |
| Instrument specification(s) | AmCyan channel |
| Fluorescence microscope | |
| Excitation | Violet filter |
| Emission | Violet filter |
| Recommended plate | Black wall/clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 17, 2026
