Cell Meter™ PE-Annexin V Binding Apoptosis Assay Kit
Optimized for Flow Cytometry
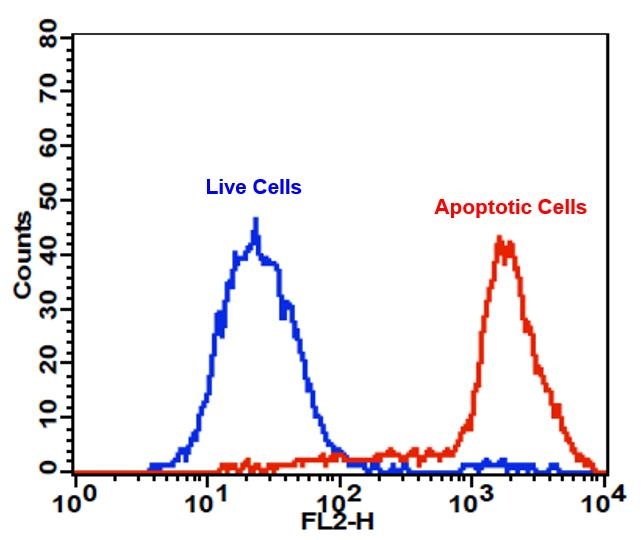
Annexin V may be conjugated to fluorochromes including PE. This format retains its high affinity for phosphatidylserine (PS) and thus serves as a sensitive probe for flow cytometric analysis of cells that are undergoing apoptosis. Since externalization of PS occurs in the earlier stages of apoptosis, PE Annexin V staining can identify apoptosis at an earlier stage than assays based on nuclear changes such as DNA fragmentation. PE Annexin V staining precedes the loss of membrane integrity which accompanies the latest stages of cell death resulting from either apoptotic or necrotic processes. Therefore, staining with PE Annexin V is typically used in conjunction with a vital dye such as propidium iodide (PI) or 7-Amino-Actinomycin (7-AAD) to allow the investigator to identify early apoptotic cells (7-AAD negative, PE Annexin V positive). Viable cells with intact membranes exclude 7-AAD, whereas the membranes of dead and damaged cells are permeable to 7-AAD. For example, cells that are considered viable are both PE Annexin V and 7-AAD negative while cells that are in early apoptosis are PE Annexin V positive and 7-AAD negative, while cells that are in late apoptosis or already dead are both PE Annexin V and 7-AAD positive. This assay does not distinguish between cells that have undergone apoptotic death versus those that have died as a result of a necrotic pathway because in either case, the dead cells will stain with both PE Annexin V and 7-AAD. However, when apoptosis is measured over time, cells can be often tracked from PE Annexin V and 7-AAD negative (viable, or no measurable apoptosis), to PE Annexin V positive and 7-AAD negative (early apoptosis, membrane integrity is present) and finally to PE Annexin V and 7-AAD positive (end stage apoptosis and death). The movement of cells through these three stages suggests apoptosis. In contrast, a single observation indicating that cells are both PE Annexin V and 7-AAD positive, in of itself, reveals less information about the process by which the cells underwent their demise.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22838 | 100 Tests | Price |
Spectral properties
| Correction factor (280 nm) | 0.175 |
| Extinction coefficient (cm -1 M -1) | 1960000 |
| Excitation (nm) | 565 |
| Emission (nm) | 574 |
| Quantum yield | 0.82 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm or 561 nm laser |
| Emission | 575/26 nm filter |
| Instrument specification(s) | PE channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on October 8, 2024

