Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Red Fluorescence*
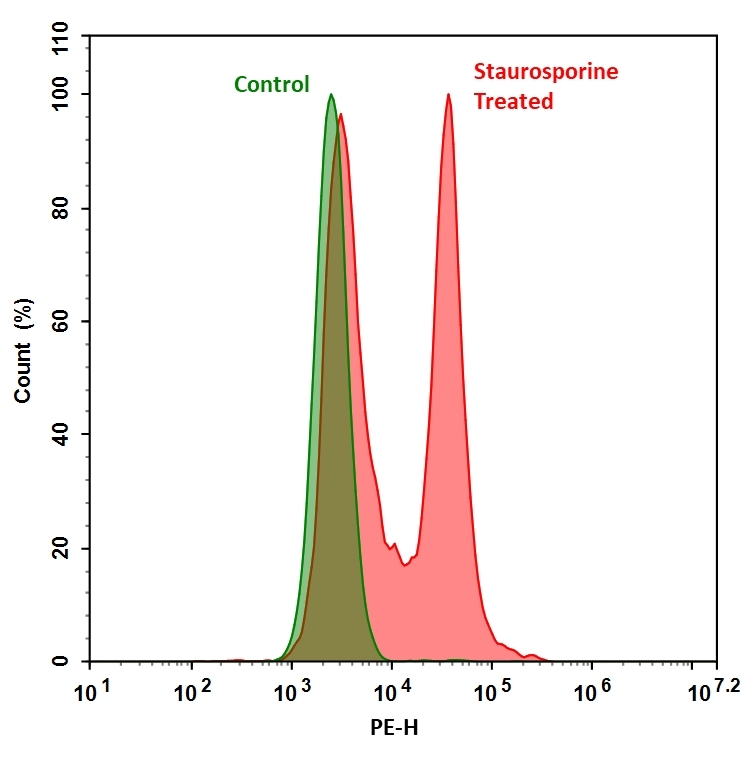
Our Cell Meter™ live cell caspases activity assay kits are based on fluorescent FMK inhibitors of caspases. These inhibitors are cell permeable and non-cytotoxic. Once inside the cell, the caspase inhibitors bind covalently to the active caspases. The activation of caspase 3/7 is important for the initiation of apoptosis. It has been proven that caspase 3/7 has substrate selectivity for the peptide sequence Asp-Glu-Val-Asp (DEVD). This kit uses TF3-DEVD-FMK as a fluorescent indicator for caspase 3/7 activity. TF3-DEVD-FMK irreversibly binds to activated caspase 3/7 in apoptotic cells. Once bound to caspase 3/7, the fluorescent reagent is retained inside the cell. The binding event inhibits caspase 3/7 but will not stop apoptosis from proceeding. There are a variety of parameters that can be used for monitoring cell apoptosis. This Cell Meter™ Live Cell Caspase 3/7 Activity Assay Kit is designed to detect cell apoptosis by measuring caspase 3/7 activation in live cells. It is used for the quantification of activated caspase 3/7 activities in apoptotic cells, or for screening caspase 3/7 inhibitors. TF3-DEVD-FMK, the red label reagent, allows for direct detection of activated caspase 3/7 in apoptotic cells by fluorescence microscopy, flow cytometer, or fluorescent microplate reader. The kit provides all the essential components with an optimized assay protocol.
Example protocol
AT A GLANCE
Protocol summary
- Prepare cells with test compounds at a density of 5 × 105 to 2 × 106 cells/mL
- Add TF3-DEVD-FMK into cell solution at 1:150 ratio
- Incubate at room temperature for 1 hour
- Pellet the cells, wash and resuspend the cells with buffer or growth medium
- Monitor fluorescence intensity (bottom read mode) at Ex/Em = 550/595 nm (Cutoff = 570 nm), fluorescence microscope with TRITC filter, or flow cytometer with FL1 channel
Important notes
Thaw all the components at room temperature before starting the experiment.
PREPARATION OF STOCK SOLUTION
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles.
1. TF3-DEVD-FMK stock solution (150X):
Add 50 µL of DMSO into the vial of TF3-DEVD-FMK (Component A) to make 150X TF3-DEVD-FMK stock solution.
For guidelines on cell sample preparation, please visit
https://www.aatbio.com/resources/guides/cell-sample-preparation.html
SAMPLE EXPERIMENTAL PROTOCOL
- Culture cells to a density optimal for apoptosis induction according to your specific induction protocol, but not to exceed 2 x 106 cells/ mL. At the same time, culture a non-induced negative control cell population at the same density as the induced population for every labeling condition. Here are a few examples for inducing apoptosis in suspension culture:
- Treating Jurkat cells with 2 µg/ml camptothecin for 3 hours.
- Treating Jurkat cells with 1 µM staurosporine for 3 hours.
- Treating HL-60 cells with 4 µg/ml camptothecin for 4 hours.
- Treating HL-60 cells with 1 µM staurosporine for 4 hours. Note: Each cell line should be evaluated on an individual basis to determine the optimal cell density for apoptosis induction.
- Treating Jurkat cells with 2 µg/ml camptothecin for 3 hours.
- Add 150X TF3-DEVD-FMK stock solution into the cell solution at a 1:150 ratio, and incubate the cells in a 37°C, 5% CO2 incubator for 1 hour. Note: The cells can be concentrated up to ~ 5 X 106 cells/mL for TF3-DEVD-FMK labeling. For adherent cells, gently lift the cells with 0.5 mM EDTA to keep the cells intact, and wash the cells once with serum-containing media prior to incubation with TF3-DEVD-FMK. The appropriate incubation time depends on the individual cell type and cell concentration used. Optimize the incubation time for each experiment.
- Spin down the cells at ~ 200g for 5 minutes, and wash cells with 1 mL Washing Buffer (Component B) twice. Resuspend the cells in desired amount of washing buffer. Note: TF3-DEVD-FMK is fluorescent, thus it is important to wash out any unbound reagent to eliminate the background. For detached cells, the concentration of cells should be adjusted to 2 - 5 X 105 cells/100 µL aliquot per microtiter plate well.
- If desired, label the cells with a DNA stain (such as Nuclear Green™ DCS1 for dead cells, or Hoechst for whole population of the cell nucleus stain).
- Monitor the fluorescence intensity by fluorescence microscopy, flow cytometer, or fluorescence microplate reader at Ex/Em = 550/595 nm (for Nuclear Green™ DCS1, Ex/Em = 490/525 nm, for Hoechst dyes, Ex/Em = 350/461 nm).
For flow cytometry: Monitor the fluorescence intensity using the channel with Ex/Em = 550/595 nm (FL1 channel for Nuclear Green™ DCS1 staining). Gate on the cells of interest, excluding debris.
For fluorescence microscope: Place 100 µL of the cell suspensions into each of wells of a 96-well black microtiter plate. Observe cells under a fluorescence microscope using TRITC channel (FITC channel for Nuclear Green™ DCS1 staining, DAPI channel for Hoechst staining).
For fluorescence microplate reader: Place 100 µL of the cell suspensions into each of wells of a 96-well black microtiter plate. Monitor the fluorescence intensity (bottom read mode) with a fluorescence microplate reader at Ex/Em = 550/595 nm (Cutoff = 570 nm). Note: If it is necessary to equilibrate the cell concentrations, adjust the suspension volume for the induced cells to approximate the cell density of the non-induced population. This adjustment step is optional if your cell treatment does not result in a dramatic loss in stimulated cell population numbers.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Correction Factor (260 nm) | Correction Factor (280 nm) |
| Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Green Fluorescence* | 493 | 517 | 83000 | 0.32 | 0.178 |
Citations
View all 12 citations: Citation Explorer
Executioner caspases degrade essential mediators of pathogen-host interactions to inhibit growth of intracellular Listeria monocytogenes
Authors: Lavergne, Marilyne and Schaerer, Raffael and De Grandis, Sara and Bouheraoua, Safaa and Adenuga, Oluwadamilola and Muralt, Tanja and Schaerer, Tiffany and Ch{\`e}vre, L{\'e}a and Failla, Alessandro and Matthey, Patricia and others,
Journal: Cell Death \& Disease (2025): 55
Authors: Lavergne, Marilyne and Schaerer, Raffael and De Grandis, Sara and Bouheraoua, Safaa and Adenuga, Oluwadamilola and Muralt, Tanja and Schaerer, Tiffany and Ch{\`e}vre, L{\'e}a and Failla, Alessandro and Matthey, Patricia and others,
Journal: Cell Death \& Disease (2025): 55
Isotretinoin promotes elimination of translation-competent HIV latent reservoirs in CD4T cells
Authors: Howard, J Natalie and Levinger, Callie and Deletsu, Selase and Fromentin, R{\'e}mi and Chomont, Nicolas and Bosque, Alberto and AIDS Clinical Trials Group (ACTG) A5325 Team,
Journal: PLoS pathogens (2024): e1012601
Authors: Howard, J Natalie and Levinger, Callie and Deletsu, Selase and Fromentin, R{\'e}mi and Chomont, Nicolas and Bosque, Alberto and AIDS Clinical Trials Group (ACTG) A5325 Team,
Journal: PLoS pathogens (2024): e1012601
Executioner caspases degrade essential mediators of pathogen-host interactions to inhibit growth of intracellular Listeria monocytogenes
Authors: Walch, Michael and Lavergne, Marilyne and Schaerer, Raffael and Bouheraoua, Safaa and Adenuga, Oluwadamilola and Muralt, Tanja and Schaerer, Tiffany and Ch{\`e}vre, L{\'e}a and Failla, Alessandro and Matthey, Patricia and others,
Journal: (2024)
Authors: Walch, Michael and Lavergne, Marilyne and Schaerer, Raffael and Bouheraoua, Safaa and Adenuga, Oluwadamilola and Muralt, Tanja and Schaerer, Tiffany and Ch{\`e}vre, L{\'e}a and Failla, Alessandro and Matthey, Patricia and others,
Journal: (2024)
Investigating the Molecular Mechanisms of TMEM16F--a Ca2+ Activated Phospholipid Scramblase and Ion Channel
Authors: Le, Trieu Phuong Hai
Journal: (2021)
Authors: Le, Trieu Phuong Hai
Journal: (2021)
Evidence that polyphenols do not inhibit the phospholipid scramblase TMEM16F
Authors: Le, Trieu and Le, Son C and Zhang, Yang and Liang, Pengfei and Yang, Huanghe
Journal: Journal of Biological Chemistry (2020): 12537--12544
Authors: Le, Trieu and Le, Son C and Zhang, Yang and Liang, Pengfei and Yang, Huanghe
Journal: Journal of Biological Chemistry (2020): 12537--12544
References
View all 50 references: Citation Explorer
In vitro effect of different mediators of apoptosis on canine cranial and caudal cruciate ligament fibroblasts and its reversibility by pancaspase inhibitor zVAD.fmk
Authors: Forterre S, Zurbriggen A, Spreng D.
Journal: Vet Immunol Immunopathol (2011): 264
Authors: Forterre S, Zurbriggen A, Spreng D.
Journal: Vet Immunol Immunopathol (2011): 264
Intracochlear perfusion of leupeptin and z-VAD-FMK: influence of antiapoptotic agents on gunshot-induced hearing loss
Authors: Abaamrane L, Raffin F, Schmerber S, Sendowski I.
Journal: Eur Arch Otorhinolaryngol (2011): 987
Authors: Abaamrane L, Raffin F, Schmerber S, Sendowski I.
Journal: Eur Arch Otorhinolaryngol (2011): 987
Structure of human caspase-6 in complex with Z-VAD-FMK: New peptide binding mode observed for the non-canonical caspase conformation
Authors: Muller I, Lamers MB, Ritchie AJ, Dominguez C, Munoz-Sanjuan I, Kiselyov A.
Journal: Bioorg Med Chem Lett (2011): 5244
Authors: Muller I, Lamers MB, Ritchie AJ, Dominguez C, Munoz-Sanjuan I, Kiselyov A.
Journal: Bioorg Med Chem Lett (2011): 5244
Experimental study on treatment of rabbits optic nerve injury with Caspase-3 inhibitor z-DEVD-fmk
Authors: Zhang W, Yu JG, Wang X, Shen ZS, Zhang JK, Yan H.
Journal: Zhonghua Yan Ke Za Zhi (2010): 1084
Authors: Zhang W, Yu JG, Wang X, Shen ZS, Zhang JK, Yan H.
Journal: Zhonghua Yan Ke Za Zhi (2010): 1084
Caspase inhibitor ZVAD-fmk facilitates engraftment of donor hematopoietic stem cells in intra-bone marrow-bone marrow transplantation
Authors: Imai Y, Adachi Y, Shi M, Shima C, Yanai S, Okigaki M, Yamashima T, Kaneko K, Ikehara S.
Journal: Stem Cells Dev (2010): 461
Authors: Imai Y, Adachi Y, Shi M, Shima C, Yanai S, Okigaki M, Yamashima T, Kaneko K, Ikehara S.
Journal: Stem Cells Dev (2010): 461
Page updated on March 11, 2025