Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit
Green Fluorescence
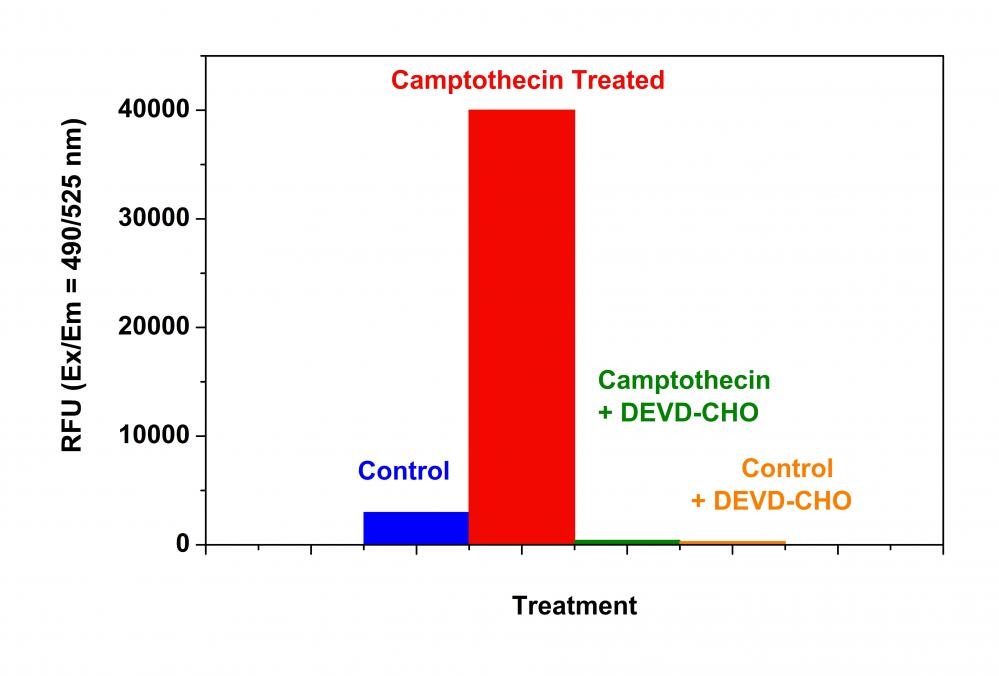
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used for monitoring cell viability. This particular kit is designed to monitor cell apoptosis through measuring Caspase 3 activation. Caspase 3 is widely accepted as a reliable indicator for cell apoptosis since the activation of caspase-3 (CPP32/apopain) is important for the initiation of apoptosis. Caspase 3 has substrate selectivity for the peptide sequence Asp-Glu-Val-Asp (DEVD). This kit uses Z-DEVD-Rh 110-DVED-Z as a fluorogenic indicator for caspase-3 activity. Cleavage of Rh 110 peptides by caspase 3 generates strongly fluorescent Rh 110 that is monitored fluorimetrically at 520-530 nm with excitation of 480-500 nm. The kit provides all the essential components with an optimized assay protocol. The assay is robust, and can be readily adapted for high-throughput assays. Using 100 uL of reagents per well in a 96-well format, this kit provides sufficient reagents to perform 200 assays. Using 25 uL of reagents per well in a 384-well format, this kit provides sufficient reagents to perform 800 assays.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22796 | 200 Tests | Price |
Spectral properties
| Extinction coefficient (cm -1 M -1) | 80000 |
| Excitation (nm) | 500 |
| Emission (nm) | 522 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Top/Bottom read mode |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 13, 2026

