Cell Meter™ Generic Fluorimetric Caspase Activity Assay Kit
Green Fluorescence Optimized for Flow Cytometry
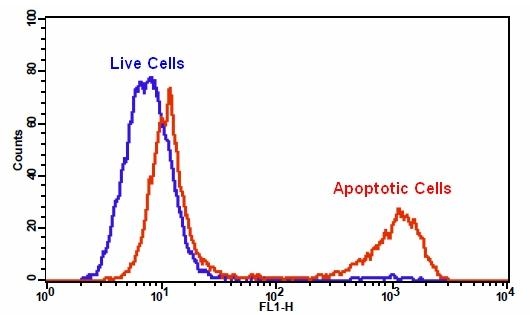
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used for monitoring cell viability. This particular kit is designed to monitor cell apoptosis through measuring generic caspases (caspase-1, -3, -4, -5, -6, -7, -8 and -9) activation in living cells. Caspases activation is widely accepted as a reliable indicator for cell apoptosis. Most caspases have substrate selectivity for the peptide sequence Val-Ala-Asp (VAD). This kit uses TF2-VAD-FMK as a fluorogenic indicator for most caspase activity. TF2-VAD-FMK is cell permeable, nontoxic, and irreversibly binds to activated casepase-1, -3, -4, -5, -6, -7, -8 and -9 in apoptotic cells. Once bound to caspases, the green fluorescent reagent is retained within the cell. The binding event prevents the caspases from further catalysis but will not stop apoptosis from proceeding. The reagent will start to react with active caspase enzymes within 15 minutes of addition to the media. The kit provides all the essential components with an optimized assay protocol. It is used for the quantification of most activated caspases activities in apoptotic cells, or for screening caspases inhibitors. The green label allows for direct detection of activated caspases in apoptotic cells by flow cytometry.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22821 | 100 Tests | Price |
Spectral properties
| Correction factor (280 nm) | 0.09 |
| Extinction coefficient (cm -1 M -1) | 75000 |
| Excitation (nm) | 503 |
| Emission (nm) | 525 |
| Quantum yield | 0.9 1 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 488 nm laser |
| Emission | 530/30 nm filter |
| Instrument specification(s) | FITC channel |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 11, 2026

