Cell Meter™ Colorimetric WST-8 Cell Quantification Kit
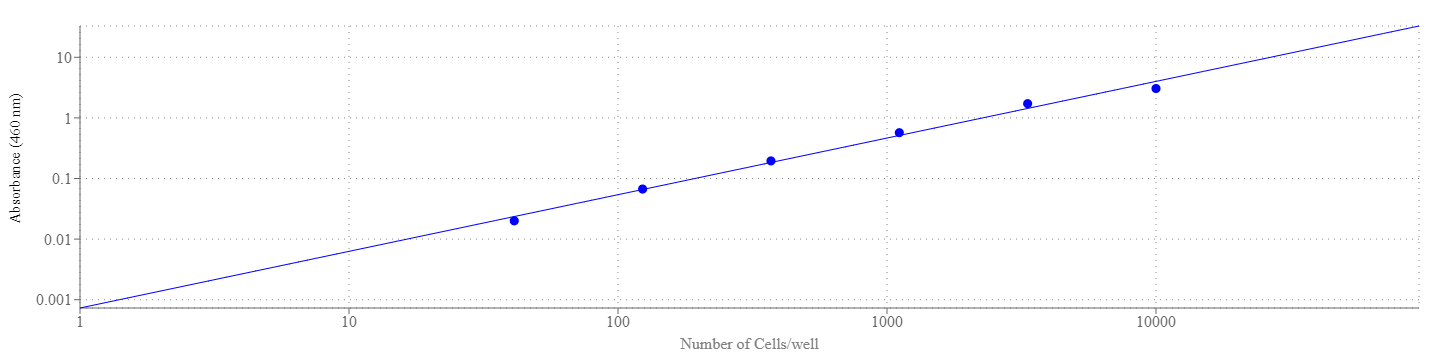
Our Cell Meter™ assay kits are a set of tools for monitoring cell viability. There are a variety of parameters that can be used for monitoring cell viability. Cell Meter™ Colorimetric WST-8 Cell Quantification Kit uses water-soluble WST-8 tetrazolium salt to quantify the number of live cells. The water-soluble WST-8 tetrazolium salt produces a water-soluble orange formazan dye upon bioreduction in the presence of an electron carrier, 1-methoxy-5-methylphenazinium methyl sulfate. The kit is convenient and robust with a mix and read format. WST-8 solution is added directly to the test cells, no pre-mixing of components is required. WST-8 tetrazolium salt is reduced by cellular dehydrogenases to an orange formazan product that is soluble in tissue culture medium. The amount of formazan produced is directly proportional to the number of living cells by monitoring absorbance increase at 460 nm. The excellent stability and little cytotoxicity of WST-8 solution make the kit useful for the assays that require long incubation (such as 24 to 48 hours). Cell Meter™ Colorimetric WST-8 Cell Quantification Kit provides a sensitive colorimetric assay for the determination of the number of viable cells in the proliferation and cytotoxicity assays. The detection sensitivity is higher than any other tetrazolium salt-based assays such as MTT, XTT or MTS etc.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 22770 | 1000 Tests | Price | |
| 22771 | 5000 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 460 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 25, 2026
