Calbryte™ 630 AM
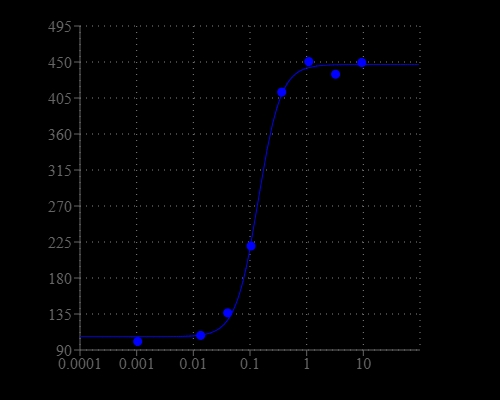
Calcium measurement is critical for numerous biological investigations. Fluorescent probes that show spectral responses upon binding calcium have enabled researchers to investigate changes in intracellular free calcium concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. x-Rhod-1 is commonly used as a red fluorescent calcium indicator. However, x-Rhod-1 is only moderately fluorescent in live cells upon esterase hydrolysis, and has very small cellular calcium responses. Calbryte™ 630 has been developed to improve x-Rhod-1 cell loading and calcium response while maintaining the spectral wavelength of x-Rhod-1, making it compatible with Texas Red® filter set. In CHO and HEK cells Cal-630™ AM has cellular calcium response that is much more sensitive than x-Rhod-1. The spectra of Calbryte™ 630 is well separated from those of FITC, Alexa Fluor® 488 and GFP, making it an ideal calcium probe for multiplexing intracellular assays with GFP cell lines or FITC/Alexa Fluor® 488 labeled antibodies. Calbryte™ 630 is a new generation of red fluorescent indicators for the measurement of intracellular calcium. Its greatly improved signal/background ratio and intracellular retention properties make Calbryte™ 630 AM the most robust deep red fluorescent indicator for evaluating GPCR and calcium channel targets as well as for screening their agonists and antagonists in live cells. Like other dye AM cell loading, Calbryte™ 630 AM ester is non-fluorescent and once gets inside the cell, it is hydrolyzed by intracellular esterase and gets activated. The activated indicator is a polar molecule that is no longer capable of freely diffusing through cell membrane, essentially trapped inside cells.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 20720 | 2x50 ug | Price | |
| 20721 | 10x50 ug | Price | |
| 20722 | 1 mg | Price |
Physical properties
| Dissociation constant (Kd, nM) | 1200 |
| Molecular weight | 1234.84 |
| Solvent | DMSO |
Spectral properties
| Excitation (nm) | 607 |
| Emission (nm) | 624 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| Storage | Freeze (< -15 °C); Minimize light exposure |
| UNSPSC | 12352200 |
Instrument settings
| Flow cytometer | |
| Excitation | 640 nm laser |
| Emission | 660/20 nm filter |
| Instrument specification(s) | APC channel |
| Fluorescence microscope | |
| Excitation | Texas Red |
| Emission | Texas Red |
| Recommended plate | Black wall/clear bottom |
| Fluorescence microplate reader | |
| Excitation | 600 |
| Emission | 640 |
| Cutoff | 630 |
| Recommended plate | Black wall/clear bottom |
| Instrument specification(s) | Bottom read mode/Programmable liquid handling |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on February 5, 2026

