Amplite® Fluorimetric Neuraminidase Assay Kit
Blue Fluorescence
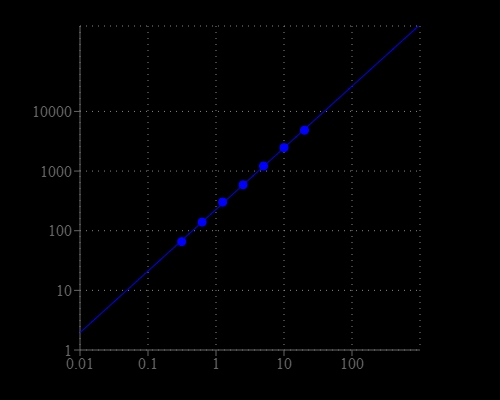
Neuraminidases, also called sialidases, are glycoside hydrolase enzymes that catalyze the hydrolysis of terminal sialic acid residues and neuraminic acid. The most commonly known neuraminidase is the viral neuraminidase. The cleavage of linkage between sialic acid and adjacent sugar residue permits the transport of the virus through mucin and destroys the haemagglutinin receptor on the host cell, thus allowing elution of progeny virus particles from infected cells. Neuraminidase promotes influenza virus release from infected cells and facilitates virus spread within the respiratory tract. Thus, it is an important target for influenza drug development. The detection of neuraminidase and screening its inhibitors is one of the essential tasks for investigating biological processes and prevention of influenza infection. There are a few assay kits available for detecting neuraminidase, but all the commercial available kits are tedious to use. Our Amplite® Fluorimetric Neuraminidase Assay Kit provides a sensitiveand robust fluorimetric assay to detect neuraminidase that exists either in cells or biological samples. The non-fluorescent neuraminidase substrate becomes strongly fluorescent upon neuraminidase cleavage. The kit can detect as little as 0.3 mU/mL neuraminidase in a 100 µL assay volume. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation without a separation step. The signal can be easily read by a fluorescence microplate reader.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 12602 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 320 nm |
| Emission | 460 nm |
| Cutoff | 420 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 28, 2026

