Amplite® Fluorimetric Maleimide Quantitation Kit
Green Fluorescence
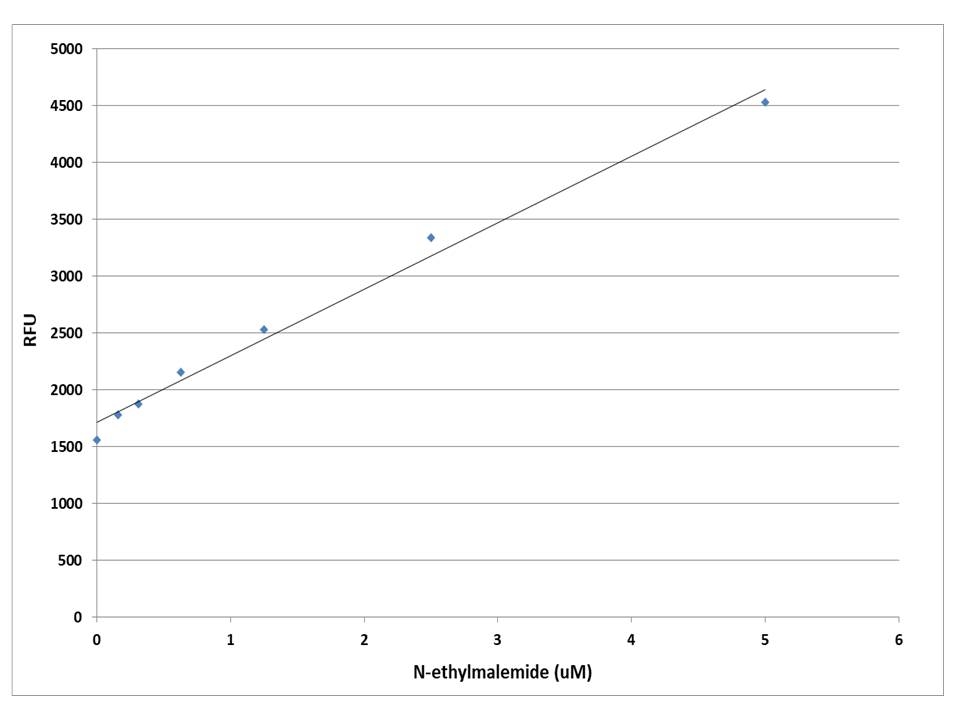
A variety of crosslinking reagents with a maleimide group are widely used for crosslinking proteins to proteins or proteins to other biomolecules. There are few reagents or assay kits available for quantitate the number of maleimide groups that are introduced into the first protein. All the commercial kits have tedious protocols. Our kit uses a proprietary dye that has enhanced fluorescence upon reacting with a maleimide. The kit provides a sensitive, one-step fluorimetric method to detect as little as 10 picomole of maleimide in a 100 µL assay volume (100 nM in concentration). The assay is rapid and robust. It can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation. The kit provides a convenient protocol with all the essential reagents. For the rapid quantification of a protein maleimide group we recommend you use #5526. For the quantification of a nano particle maleimide group we recommend you use #5525.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 5523 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 490 nm |
| Emission | 525 nm |
| Cutoff | 515 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 27, 2026
