Amplite® Fluorimetric Hypochlorite (Hypochlorous Acid) Assay Kit
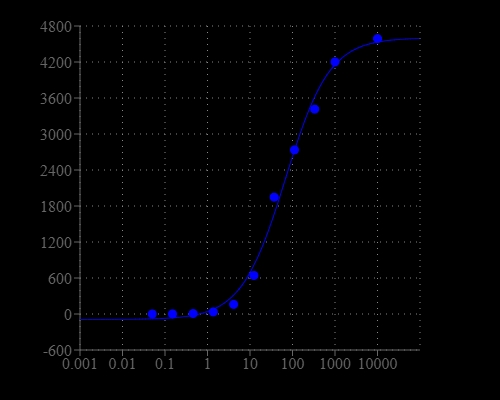
Hypochlorite anion (ClO-) and its protonated form, hypochlorous acid (HClO) are critical reactive oxygen species (ROS) in biological systems. Uncontrolled production of hypochlorite (hypochlorous acid) can lead to tissue damage and diseases including arthritis, renal failure and cancers. In addition, sodium hypochlorite (NaClO) has been widely used as a bleaching agent for surface cleaning, odor removal and water disinfection in our daily lives. Exposure to large amount of sodium hypochlorite can lead to poisoning with the symptoms of serious breathing problems, stomach irritation, redness and pain on skin and eye. Therefore, highly selective and sensitive detection of hypochlorite (hypochlorous acid) is of toxicological and environmental importance. Amplite® Fluorimetric Hypochlorite (Hypochlorous Acid) Assay Kit offers a sensitive fluorescence-based assay for measuring hypochlorite (hypochlorous acid) with high specificity. Upon selective reaction with hypochlorite (hypochlorous) the weakly fluorescent Oxirite™ Hypochlorite Sensor generates a strongly fluorescent product that gives more than 100-fold fluorescence enhancement. The fluorescence signal can be measured by a fluorescence microplate reader. With this Fluorimetric Hypochlorite (Hypochlorous Acid) Assay Kit, as low as 0.4 µM hypochlorite was detected in a 100 µL reaction volume.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13846 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 555 |
| Emission (nm) | 576 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 22, 2026

