Amplite® Fluorimetric Catalase Assay Kit
Red Fluorescence
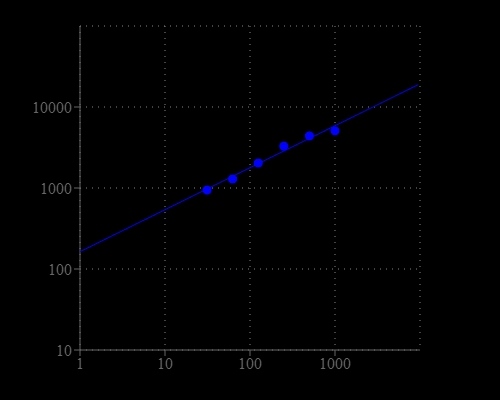
Catalase is a common antioxidant heme-containing redox enzyme found in nearly all living organisms that are exposed to oxygen. The enzyme is concentrated in the peroxisome subcellular organelles. Hydrogen peroxide is an ROS that is a toxic product of normal aerobic metabolism and pathogenic ROS production involving oxidase and superoxide dismutase reactions. By preventing the excessive buildup of H2O2, catalase allows important cellular processes which produce H2O2 as a by-product to take place safely. The Amplite® Fluorimetric Catalase Assay Kit provides a quick and sensitive method for the measurement of catalase activity. It can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation without a separation step. Catalase reacts with H2O2 to produce water and oxygen (O2). Amplite® Red also reacts with H2O2 to generate a red fluorescent product. Therefore the reduction in fluorescence intensity is proportional to catalase activity. The Amplite® Red substrate used in the assay enables a dual recordable mode. The fluorescent signal can be easily read by either a fluorescence microplate reader or an absorbance microplate reader. With the Amplite® Fluorimetric Catalase Assay Kit, we have detected as little as 30 mU/mL catalase in a 100 µL reaction volume.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11306 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 14, 2026

