Amplite® Colorimetric Urea Quantitation Kit
Blue Color
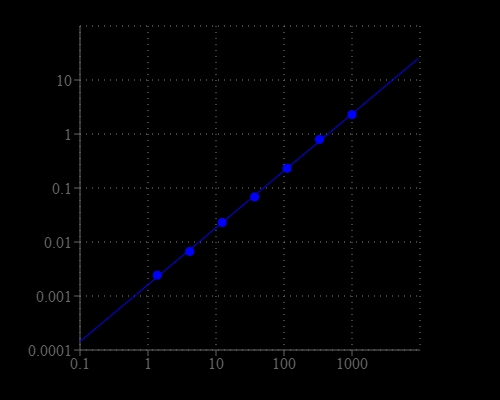
Urea is the final degradation product of protein and amino acid metabolism in animals. It is produced in liver, secreted by kidney and excreted through urine. The determination of urea is very useful test in clinical laboratory to monitor health status. The Blood Urea Nitrogen (BUN) test is a measure of the amount of nitrogen in the blood in the form of urea and is primarily used, along with the creatinine test to evaluate kidney function, helping diagnose kidney diseases. Our Amplite® Colorimetric Urea Assay Kit provides a simple and sensitive colorimetric method for the quantitation of urea concentration in biological samples such as serum, plasma and urine, etc. The assay is based on an enzyme-coupled reaction of urea in the assay buffer, and finally produces a blue colored product. The intensity of color produced is proportional to the concentration of urea in the sample, which can be measured colorimetrically at 660-670 nm. This Amplite® Colorimetric Urea Assay Kit provides a simple assay to detect as little as 10 µM urea in a 150 µL assay volume. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and easily adapted to automation without a separation step.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 10058 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 665 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 28, 2026
